2432
Automated assessment of longitudinal White Matter Hyperintensities changes using a novel convolutional neural network in CADASIL
Valentin Demeusy1, Florent Roche1, Fabrice Vincent1, Jean-Pierre Guichard2, Jessica Lebenberg3,4, Eric Jouvent3,5, and Hugues Chabriat3,5
1Imaging Core Lab, Medpace, Lyon, France, 2Department of Neuroradiology, Hôpital Lariboisière, APHP, Paris, France, 3FHU NeuroVasc, INSERM U1141, Paris, France, 4Université de Paris, Paris, France, 5Departement of Neurology, Hôpital Lariboisière, APHP, Paris, France
1Imaging Core Lab, Medpace, Lyon, France, 2Department of Neuroradiology, Hôpital Lariboisière, APHP, Paris, France, 3FHU NeuroVasc, INSERM U1141, Paris, France, 4Université de Paris, Paris, France, 5Departement of Neurology, Hôpital Lariboisière, APHP, Paris, France
Synopsis
We propose a novel automatic WMH segmentation method based on a convolutional neural network to study the longitudinal WMH changes among a cohort of 101 CADASIL patients. We demonstrate that this method is able to produce consistent quantitative measures of WMH volume by the strong correlation between the computed baseline WMH volume and the clinically assessed Fazekas score. Our main results show that the progression of WMH is correlated to the baseline volume and that this progression largely vary at individual level although a rapid extension is mainly detected between 40 and 60 years in the whole population.
Introduction
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is the most frequent hereditary cerebral small vessel disease (CSVD). The disease is caused by the accumulation of extracellular-domains of the mutant NOTCH3 protein within the wall of cerebral arterioles and capillaries. CADASIL is a severe condition that occurs during mid-adulthood and can lead to stroke, mood disturbances, motor disability and cognitive decline up to severe dementia. No preventative treatment is available yet. MR imaging reveals in all affected individuals white-matter hyperintensities (WMH) on T2-weighted or FLAIR images since the early stage of the disease and developing with the disease progression. Quantification and longitudinal tracking of WMH changes in CADASIL will be of crucial importance to evaluate future disease treatments. The task is challenging due to major signal changes observed in the cerebral tissue with aging with the disease. Herein, we propose an innovative, accurate and reproducible convolutional neural network (CNN) to track WMH along disease progression.Methods
A sample of 101 CADASIL patients of age ranging from 24 to 74 years at date of MRI exams was selected from a large cohort of CADASIL patients evaluated over 15 years in the French National Referral center for Rare Vascular diseases of the Brain and Retina (www.cervco.fr). During follow-up, we monitored each patient using MRI from three to 10 times, every 18 months, starting from the first exam (baseline). The MRI protocol included 3D T1 (1.0x1.0x0.8mm3) and 2D T2-weighted FLAIR (0.46x0.46x5.5mm3) images. Expert clinicians and radiologists first segmented WMH using 2D FLAIR images at baseline and scored WMH using the Fazekas1 scale. Thereafter, we developed an automatic WMH segmentation method using a CNN 2,3 . In order to train this network, we split our data into a training (32%), validation (13%) and test (55%) sets. Epoch and threshold selection were performed by optimizing the results over the validation set. The final performance of the model was assessed using the Dice score on the test set only. When needed, the intracranial volume was obtained using ANTs 4 skull strip and the whole brain segmentation by FreeSurfer 5.Results
The main characteristics of our patients from test dataset are summarized in table 1. We first confirmed that the extent of WMH measured by the Fazekas score was correlated to the age and WMH volume assessed by our method but not to the intracranial volume or the whole brain volume; correlations score are recorded in table 2. Our CNN model reached a mean Dice index of 0.84 over the test (visual comparison on figure 3), while the reported inter-reader Dice index3 was 0.805. Previous work6 reported a Dice index of 0.80 using BIANCA7 in semi-automatic pipeline. Using our method, we found that the WMH volumes obtained at baseline were strongly correlated to the Fazekas scores (Spearman r=0.921; p-value < 0.001) (c.f. figure 1). Our main results showed a large variability of WMH changes at individual level with some patients showing a large progression between 40 and 60 years of age while others did not change even after 60 years. We found that the increase of WMH during follow-up was larger in patients with Fazekas score between 3 and 5 (average progression of over 3.8mL/years) than in patients with Fazekas score at 2 (average progression of 1.3mL/years). The results also showed a possible plateau at early (Fazekas 1) and late (Fazekas 6) stages of the disorder where the changes are the smallest (see figure 2).Discussion
The results obtained using automated measures of WMH appear promising. In the present study, our data showed that this innovative approach allows obtaining measures correlated to semi-quantitative visual measures and that the progression of WMH along follow-up was strongly correlated to these baseline measures as already and repeatedly demonstrated in CSVD. But the results also suggest that the progression of WMH largely vary at individual level further suggesting that we might have a group of fast and another of slow ‘progressors’ during the course of the disease. There are limitations in this study; 1) the sample of CADASIL patients remains relatively limited, 2) the progression of WMH was not compared to a reference method, 3) the results were obtained using 2D FLAIR images which are not well suited for volume assessment due to their anisotropy and can lead to noisy intra-subject measures, 4) additional studies are needed for evaluating potential correlations with clinical manifestations, taking into account the number of lacunes, microbleeds and degree of atrophy. In contrast, we can also consider that in this study we included a large number of repeated MRI measures in patients with a rare but archetypal CSVD, that the overall trends were easily delineated and that our results appear consistent with what we already learned from the natural history of CADASIL.Conclusion
Using a segmentation method based on CNN, we obtained consistent quantitative volumetric measures of WMH in CADASIL patients. Moreover, we measured their longitudinal changes during short or long follow-up on repeated MRI examinations. The results showed that the progression of WMH can largely vary at individual level although a rapid extension is mainly detected between 40 and 60 years in the whole population.Acknowledgements
This work was supported by grant from the National Research Agency, France (ANR-16-RHUS-0004 [RHU TRT_cSVD]).References
- Fazekas, F., Barkhof, F., Wahlund, L., Pantoni, L., Erkinjuntti, T., Scheltens, P., & Schmidt, R. (2002). CT and MRI Rating of White Matter Lesions. Cerebrovasc Dis, 31-36. doi:10.1159/000049147
- Szegedy, C., Ioffe, S., Vanhoucke, V., & Alemi, A. (n.d.). Inception-v4, Inception-ResNet and the Impact of Residual Connections on Learning. Proceedings of the Thirty-First AAAI Conference on Artificial Intelligence.
- Ghafoorian, M., Karssemeijer, N., Heskes, T., van Uden, I. W., Sanchez, C. I., Litjens, G., . . . Platel, B. (2017). Location Sensitive Deep Convolutional Neural Networks for Segmentation of White Matter Hyperintensities. Nature.
- Avants, B., Tustison, N., &
Song, G. (2009). Advanced normalization
tools (ANTS). Insight Journal.
- Fischl, B. (2012). FreeSurfer. NeuroImage.
- Ling, Y., Jouvent, E., Cousyn, L., Chabriat, H., & De Guio, F. (2018). Validation and Optimization of BIANCA for the Segmentation of Extensive White Matter Hyperintensities. Neuroinformatics.
- Griffanti, L., Zamboni, G., Khan, A., Li, L.,
Bonifacio, G., Sunderesan, V., . . . Jenkinson, M. (2016). BIANCA (Brain
Intensity AbNormality Classification Algorithm): A new tool for automated segmentation
of white matter hyperintensities. Neuroimage.
Figures
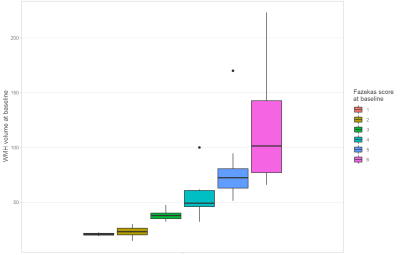
Figure 1: WMH volume per Fazekas score; both evaluated at baseline. WMH volume is highly correlated to the Fazekas score as shown by the clear separation of the different classes in the boxplot and the Spearman correlation of 0.921 (p-value < 0.001).

Figure 2: WMH volume evolution for each subject according to age. Each subject was attributed a Fazekas score at baseline. This demonstrates the variable growth of the WMH for each subject even for those with an older age.

Table 1: Statistics at
baseline for each Fazekas group over the test set showing its diversity; from
left to right: number of subject , mean and standard deviation of the age, WMH volume, intracranial volume (ICV) and whole brain volume (WBV).

Table 2: Spearman correlation between the Fazekas score assessed at baseline and the following measures, all done at the baseline : age, white matter hyperintensities (WMH) volume, intracranial volume (ICV), whole brain volume (WBV). The corresponding p-values are given in the third column.
Figure 3: Comparison between manual segmentation (middle) and our segmentation using convolutional neural network (right) on a 2D FLAIR (left) for a patient from the test set.