2411
Development of a Deep Learning MRI Phase Unwrapping Technique Using an Exact Unwrap-Rewrap Training Model
Rita Kharboush1, Anita Karsa1, Barbara Dymerska1, and Karin Shmueli1
1Medical Physics and Biomedical Engineering, University College London, London, United Kingdom
1Medical Physics and Biomedical Engineering, University College London, London, United Kingdom
Synopsis
No existing phase unwrapping technique achieves completely accurate unwrapping. Therefore, we trained a convolutional neural network for phase unwrapping on (flipped and scaled) brain images from 12 healthy volunteers. An exact model of phase unwrapping was used: ground-truth (label) phase images (unwrapped with an iterative Laplacian Preconditioned Conjugate Gradient technique) were rewrapped (projected into the 2π range) to provide input images. This novel model can be used to train any neural network. Networks trained using masked (and unmasked) images showed unwrapping performance similar to state-of-the-art SEGUE phase unwrapping on test brain images and showed some generalisation to pelvic images.
Introduction
MR phase is essential for several applications such as image distortion correction, susceptibility mapping, blood flow and temperature measurements1-5. The ‘raw’ phase is the projection of the true phase into the 2π range. This results in phase discontinuities (wraps) that need to be unwrapped. Current unwrapping techniques often compromise on accuracy or robustness against noise or have pre-processing requirements6-7. New deep learning (DL) approaches are being developed such as a bidirectional Recurrent Neural Network (b-RNN) method8 trained on maps unwrapped using PRELUDE9, the current gold-standard unwrapping technique. However, even PRELUDE solutions can contain residual wraps, meaning b-RNN solutions can surpass PRELUDE in computational speed but not in accuracy. Here, we used a novel, exact model of MRI phase unwrapping to train a convolutional neural network (CNN), aiming to obtain accurate unwrapped phase solutions. Unwrapped MRI phase images were used as a ground truth (label) and the projection of each into the 2π range was used as the wrapped input phase. We investigated the impact of CNN hyperparameters on machine learning, tested the CNN on un-seen in-vivo brain and pelvic scans, and compared its performance with a state-of-the-art unwrapping technique (SEGUE12).Methods
CNN ArchitectureWe adopted a convolutional neural network (CNN) with a 3D, multi-layered U-Net architecture originally designed for quantitative susceptibility mapping: QSMnet10-11 (Figure 1).
Training Strategy
Training, validation, and test data were acquired in fifteen healthy volunteers13 on a Magnetom Biograph mMR PET/MRI system (Siemens Healthcare, Erlangen), using a 12-channel head coil, a 3D gradient-echo sequence, TE=19.7 ms, TR=27 ms, BW=140 Hz/Pixel, α=15°, and resolution=0.9x0.9x1.5 mm3. The training dataset (12 volunteers) was augmented by flipping each image across the x, y, and z axes separately, and multiplying by factors of 1.5, 2 and 3, resulting in 192 training image pairs. Data from the remaining 3 volunteers was used for testing.
To create an exact model for training, all training phase images were unwrapped with an iterative Laplacian Preconditioned Conjugate Gradient (PCG) technique7 to provide a ground truth (label) which was projected into the 2π range to give the rewrapped input phase (Figure 2). This ensured that the label was the exact unwrapping solution for the input. The training was done using small patches of both unmasked and masked datasets to investigate the effect of masking on CNN unwrapping. Brain masks were created by adding the grey matter, white matter, and CSF regions obtained using the SPM Segment toolbox14.
We optimised the patch size, learning rate, batch size, and cost function. CNN unwrapping solutions for both ‘raw’ and re-wrapped phase images were compared to the SEGUE12 solutions.
Model Generalisation
To examine the model’s ability to generalise to images of different anatomy acquired with different parameters than the training dataset, the trained CNN was tested on multi-echo in-vivo pelvic scans acquired in one healthy volunteer by Bray et al. 15, 16 at 3T (Ingenia, Philips Healthcare, NL). The images were acquired through the sacroiliac joints (parallel to the long axis of the sacrum) using a 3D spoiled gradient‐echo pulse sequence with monopolar readout gradients, with integrated posterior and anterior surface coils (each with 16 channels) FOV=50×50×80 cm3, resolution=1.56×1.56×2 mm3, TE1=1.17 ms, ΔTE=1.6 ms, 6 echoes, TR=25 ms, α=3°, and bandwidth=1894 Hz/pixel.
Results
The hyperparameters that gave the best unwrapping solutions were found to be a 64x64x64 patch, 0.01 learning rate, 8-image batch size, and an L1-norm cost function. Figures 3 and 4 show the performance of the CNNs trained using both masked and unmasked models in comparison to SEGUE on a modelled, re-wrapped phase image and on a raw phase image (also compared to Laplacian PCG unwrapping), respectively. The network trained on unmasked images performed similarly to SEGUE. The network trained on masked images achieved the closest solutions to the ground truth. On raw phase images (Figure 4), the CNN trained on masked images gave unwrapping results comparable to Laplacian PCG or SEGUE, improving the unwrapping in some regions (yellow arrows) but introducing errors in others (red arrows). Figure 5 shows that the CNN unwrapped most of the pelvic phase image at the first echo but was unsuccessful near the edges. The CNN was less successful than SEGUE in unwrapping images at later echo times and more successful than SEGUE in unwrapping close to the sacrum.Discussion and conclusion
We have developed a universal phase unwrapping training model which can be used to train any neural network architecture to generate fast and robust solutions to the phase unwrapping problem. Using this exact model of phase unwrapping, we have trained a convolutional neural network to unwrap unmasked and masked phase images. Both trained networks achieved promising performance when compared to SEGUE on re-wrapped test images, and unwrapping performance similar to SEGUE on raw MRI phase images. CNN unwrapping in pelvic images with different acquisition parameters to the training data shows promise for the generalisability of the network, but further training with more diverse training data is needed for accurate unwrapping across different anatomical regions and acquisition protocols.Acknowledgements
We thank Prof. Jonathan Schott for permission to use images acquired as part of Insight 46, a neuroscience sub-study of the MRC National Survey of Health and Development, to train our network. Dr Karin Shmueli and Dr Anita Karsa are supported by European Research Council Consolidator Grant DiSCo MRI SFN 770939. Dr Barbara Dymerska was supported by EU Marie Skłodowska‐Curie Action MRI COMIQSUM 798119.References
- C. Liu, W. Li, K. A. Tong, K. W. Yeom, and S. Kuzminski, ‘Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain: Brain Susceptibility Imaging and Mapping’, J. Magn. Reson. Imaging, vol. 42, no. 1, pp. 23–41, Jul. 2015, doi: 10.1002/jmri.24768.
- P. Jezzard and R. S. Balaban, ‘Correction for geometric distortion in echo planar images from B0 field variations’, Magn. Reson. Med., vol. 34, no. 1, pp. 65–73, Jul. 1995, doi: 10.1002/mrm.1910340111.
- K. Shmueli, J. A. de Zwart, P. van Gelderen, T.-Q. Li, S. J. Dodd, and J. H. Duyn, ‘Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data’, Magn. Reson. Med., vol. 62, no. 6, pp. 1510–1522, Dec. 2009, doi: 10.1002/mrm.22135.
- . D. Poorter, C. D. Wagter, Y. D. Deene, C. Thomsen, F. Ståhlberg, and E. Achten, ‘Noninvasive MRI Thermometry with the Proton Resonance Frequency (PRF) Method:In Vivo Results in Human Muscle’, Magn. Reson. Med., vol. 33, no. 1, pp. 74–81, Jan. 1995, doi: 10.1002/mrm.1910330111.
- N. J. Pelc, ‘Flow quantification and analysis methods’, Magn. Reson. Imaging Clin. N. Am., vol. 3, no. 3, pp. 413–424, Aug. 1995.
- S. D. Robinson, K. Bredies, D. Khabipova, B. Dymerska, J. P. Marques, and F. Schweser, ‘An illustrated comparison of processing methods for MR phase imaging and QSM: combining array coil signals and phase unwrapping: Phase image combination and unwrapping’, NMR Biomed., vol. 30, no. 4, p. e3601, Apr. 2017, doi: 10.1002/nbm.3601.
- D. C. Ghiglia and M. D. Pritt, Two-Dimensional Phase Unwrapping: Theory, Algorithms, and Software. 1998.
- R. Kanghyun and K. Dong-Hyun, ‘Development of a deep learning method for phase unwrapping MR images’, in ISMRM, 2019.
- M. Jenkinson, ‘Fast, automated, N-dimensional phase-unwrapping algorithm’, Magn. Reson. Med., vol. 49, no. 1, pp. 193–197, Jan. 2003, doi: 10.1002/mrm.10354.
- O. Ronneberger, P. Fischer, and T. Brox, ‘U-Net: Convolutional Networks for Biomedical Image Segmentation’, ArXiv150504597 Cs, May 2015, Accessed: Nov. 11, 2020. [Online]. Available: http://arxiv.org/abs/1505.04597
- J. Yoon et al., ‘Quantitative susceptibility mapping using deep neural network: QSMnet’, NeuroImage, vol. 179, pp. 199–206, Oct. 2018, doi: 10.1016/j.neuroimage.2018.06.030.
- A. Karsa and K. Shmueli, ‘SEGUE: A Speedy rEgion-Growing Algorithm for Unwrapping Estimated Phase’, IEEE Trans. Med. Imaging, vol. 38, no. 6, pp. 1347–1357, Jun. 2019, doi: 10.1109/TMI.2018.2884093.
- C. A. Lane, ‘Study protocol: Insight 46–a neuroscience sub-study of the mrc national survey of health and development’, BMC Neurol., vol. 17, no. 1, 2017.
- SPM - Statistical Parametric Mapping. The Wellcome Centre for Human Neuroimaging, UCL. https://www.fil.ion.ucl.ac.uk/spm/, 2020.
- Bray TJ, Bainbridge A, Punwani S, Ioannou Y, Hall‐Craggs MA. Simultaneous quantification of bone edema/adiposity and structure in inflamed bone using chemical shift‐encoded MRI in spondyloarthritis. Magn Reson Med. 2018; 79:1031–1042.
- Bray, TJP, Karsa, A, Bainbridge, A, et al. Association of bone mineral density and fat fraction with magnetic susceptibility in inflamed trabecular bone. Magn Reson Med. 2019; 81: 3094– 3107. https://doi.org/10.1002/mrm.27634
Figures
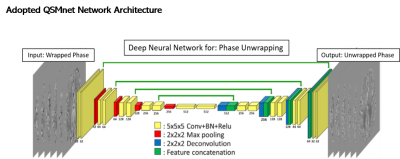
Network architecture (taken from Ref.11)
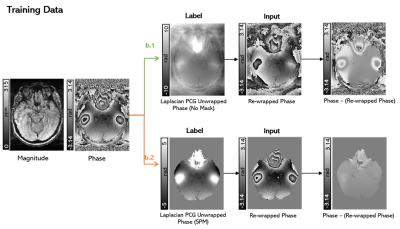
Training strategy. An
axial slice of a representative image is shown unmasked (b.1) and masked (b.2).
In all cases the label (or ground truth image) was the Laplacian PCG unwrapped
phase, and the input was the rewrapped phase of the label such that the label
was the exact unwrapping solution of the label. Differences can be seen between
the raw and the rewrapped phase images.
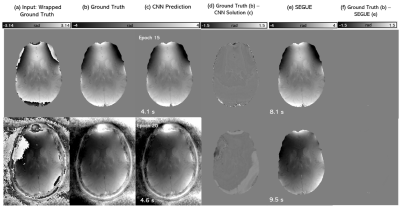
The CNN
unwrapping performance on a modelled (rewrapped) phase image. An axial slice of
the wrapped phase (a) ground truth (b) and phase unwrapping solutions using masked
CNN (c, row 1), unmasked CNN (c, row 2), CNN solutions relative to ground truth
(d), SEGUE (e), and SEGUE solutions relative to ground truth (f) for a healthy
volunteer test image. Unwrapping computation times are shown below. The lowest validation error (i.e.,
learning convergence) occurred at training epoch 15 for the masked model and at
epoch 20 for the unmasked model.
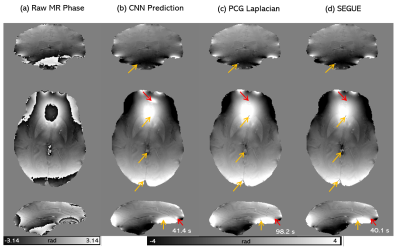
The
CNN unwrapping performance on raw phase images. A coronal, axial and sagittal
slice of the raw phase (a) and phase unwrapping solutions using the masked CNN
(b), Iterative Laplacian PCG (c) and SEGUE (d) for a representative healthy volunteer
in vivo. The computation times are shown below. Red arrows indicate where the Laplacian
or SEGUE solutions appear more accurate than the CNN. The yellow arrows
indicate where the CNN was more accurate.
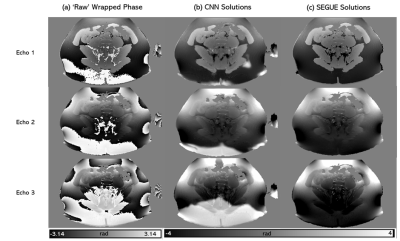
CNN
generalization study: An axial slice of a representative pelvic image with the
raw phase (a) and phase unwrapped using the network trained on masked images
(b) and SEGUE (c) at different
echo times.