2396
White and grey matter microstructural alterations and increased free-water content 13 years after very preterm birth1Victorian Infant Brain Studies (VIBeS), Murdoch Children's Research Institute, Melbourne, Australia, 2Developmental Imaging, Murdoch Children's Research Institute, Melbourne, Australia, 3Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia, 4Monash Biomedical Imaging, Monash University, Melbourne, Australia, 5Newborn Research, The Royal Women's Hospital, Melbourne, Australia, 6Department of Obstetrics and Gynaecology, The University of Melbourne, Melbourne, Australia, 7Department of Paediatrics, The University of Melbourne, Melbourne, Australia, 8Turner Institute for Brain and Mental Health and School of Psychological Sciences, Monash University, Melbourne, Australia
Synopsis
We investigated the microstructural composition of the brain tissue in 130 adolescents born very preterm (VP) compared with 45 full-term (FT)-born controls. This involved a novel voxel-based analysis of white matter-like, grey matter-like, and fluid-like (free-water) diffusion tissue signal fractions derived by Single-Shell 3-Tissue Constrained Spherical Deconvolution. VP adolescents showed widespread, diverse microstructural alterations and increased free-water content across the brain parenchyma compared with FT controls, which were associated with perinatal risk factors and adverse neurodevelopmental outcomes. This study expands knowledge of the neurobiological mechanisms by which VP birth adversely affects brain development in the long-term.
Introduction
Several studies have demonstrated that children born very preterm (VP) have striking brain white matter (WM) microstructural disturbances compared with children born full-term (FT)[1-3]. However, studies examining the microstructure of multiple brain tissue types are needed to fully capture the neurobiological mechanisms by which VP birth affects brain development and associated neurodevelopmental outcomes. Single-Shell 3-Tissue Constrained Spherical Deconvolution (SS3T-CSD) estimates signal compartments for the WM, grey matter (GM) and cerebrospinal fluid (CSF)[4]. SS3T-CSD has also recently been used to provide insight into the microstructural properties of pathological brain tissue[5,6]. In this context, tissue is characterised in terms of its relative composition of “WM-like”, “GM-like”, and “CSF-like” (free-water) diffusion signal characteristics. In this study, we investigated the microstructural and free-water composition of the whole-brain parenchyma in adolescents born VP compared with FT controls, and in relation to perinatal risk factors and concurrent neurodevelopmental outcomes. This was achieved using a voxel-based analysis (VBA) of diffusion tissue signal fractions from SS3T-CSD.Methods
At age 13 years, adolescents born VP (<30 weeks’ gestation; n=130) and FT (≥37 weeks’ gestation; n=45) underwent diffusion MRI in a 3T scanner (60 gradient directions, b=2800 s/mm2, 4 b=0 s/mm2 images, 2.4 mm isotropic voxels). Processing was predominantly performed using MRtrix3[7], MRtrix3Tissue (https://3Tissue.github.io, a fork of MRtrix3), and FSL[8]. Preprocessing included Gibbs-ringing correction[9], motion and distortion correction[10-13], estimation and averaging of 3-tissue response functions[14], upsampling to 1.5 mm3, SS3T-CSD[4], and intensity normalisation (global and bias fields) to facilitate study-specific WM fibre orientation distribution template construction[15]. Then, each participant’s WM, GM and CSF compartment images were registered to the study template[15]. Each compartment was divided by the sum of all three compartments. This generated the WM-like, GM-like and CSF-like tissue signal fraction maps, denoted as TW, TG and TC respectively (TW + TG + TC = 1)[5,6]. The reliability and long-term stability of the TW, TG and TC metrics has been documented previously[16]. The maps were smoothed (FWHM=4mm) and analysed via VBA with general linear modelling, threshold-free cluster enhancement and non-parametric permutation testing[17]. Statistical analyses were restricted to brain parenchyma (excluding the ventricles). Statistical significance was defined as p<0.05, family-wise error rate (FWE)-corrected.Results
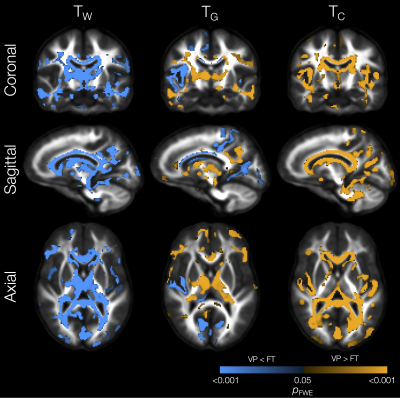
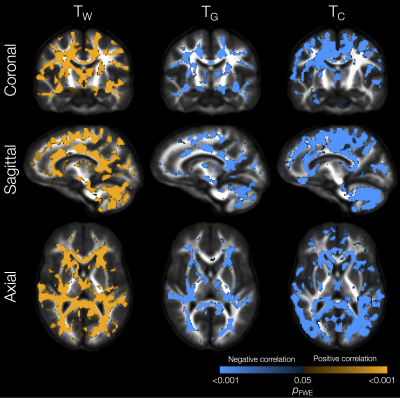
The VP group had significantly lower TW in substantial parts of the WM compared with the FT group (Figure 1). In some of those WM regions, TG was also significantly higher in the VP group, including in the internal and external capsules, cingulum, and corpus callosum. In several of the regions that had lower TW, TC was also significantly higher in the VP group, including in the corpus callosum, tapetum, optic radiation, fornix, and cerebellar white matter.In portions of the cortical GM, we observed significantly lower TG and higher TC in the VP group compared with the FT group (Figure 1). These regions included temporal (superior temporal, transverse temporal, insula), sensorimotor (precentral, paracentral), occipital (pericalcarine, lingual, lateral occipital), and cingulate cortical regions.
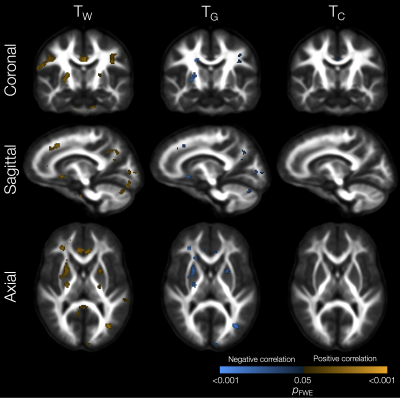
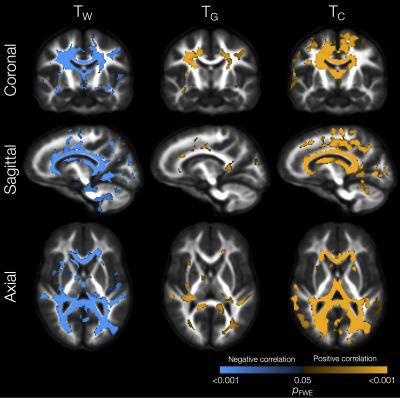
Perinatal risk factors significantly related to tissue signal fractions included lower gestational age, which was associated with lower TW and higher TG in small WM regions in the external capsule, sagittal stratum and corpus callosum (not shown). Lower birth weight z-score was related to lower TW and higher TG in some specific WM regions such as the cingulum, internal capsule, cerebellar white matter, and superior longitudinal fasciculus (Figure 2). Higher neonatal brain injury or abnormality scores[18] were associated with widespread reductions in TW and increases in TG and TC in the WM (Figure 3).
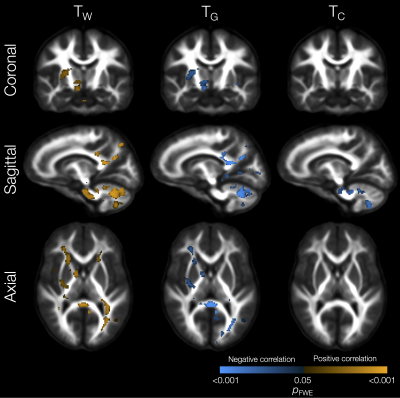
Lower TW and higher TG and TC in several WM regions (including the corpus callosum, internal and external capsules, superior and inferior longitudinal fascicules, cingulum, and cerebellar white matter) were significantly associated with lower IQ scores (Figure 4). Lower TW, higher TG and higher TC throughout the WM, and higher TC throughout the cortical GM, were significantly associated with poorer motor scores (Figure 5).
Discussion
We found widespread WM microstructural alterations in VP children compared with FT controls, in the form of a shift from the expected “WM-like” (TW) composition towards a more “GM-like” (TG) or fluid-like (TC) composition. This shift may reflect reduced axon density (TW), in line with prior reports in this population based on alternative diffusion MRI analysis methods[1-3]. The novel information provided by the current analysis is that reduced axon density may be accompanied by increases in free-water content (TC) and non-axonal cells (TG; for example, potentially glial cells). Note that TG in this context does not imply biological, functional or chemical similarity to GM, but reflects similar diffusion signal characteristics[5,6]. We also found that the VP group had cortical microstructural alterations, with a shift from a “GM-like” (TG) composition towards a more fluid-like (TC) composition. This likely reflects reduced GM cell density[19]. Furthermore, the patterns of findings support a strong link between perinatal risk factors (particularly neonatal brain abnormalities), whole-brain tissue composition alterations, and cognitive and motor impairments in VP adolescents.Conclusion
VP-born adolescents exhibit widespread, diverse microstructural alterations and increased free-water content in the brain parenchyma compared with FT-born controls, which are associated with known perinatal risk factors and adverse neurodevelopmental outcomes.Acknowledgements
We thank members of the VIBeS and Developmental Imaging teams at the Murdoch Children’s Research Institute, the Royal Children’s Hospital Medical Imaging staff for their assistance and expertise in the collection of the MRI data included in this study, and the children and families who participated. This research was supported by the Australian National Health and Medical Research Council [NHMRC; Project Grant 1066555; Centre for Research Excellence 1153176; Investigator Grant 1176077 to PA; Career Development Fellowship 1085754 to DT and 1141354 to JC], the Murdoch Children’s Research Institute, the Royal Children’s Hospital, the Royal Children’s Hospital Foundation, the Department of Paediatrics at the University of Melbourne, and the Victorian Government’s Operational Infrastructure Support Program.References
[1] Kelly CE, Thompson DK, Genc S, et al. Long-term development of white matter fibre density and morphology up to 13 years after preterm birth: A fixel-based analysis. Neuroimage. 2020;220:117068.
[2] Pannek K, Fripp J, George JM, et al. Fixel-based analysis reveals alterations is brain microstructure and macrostructure of preterm-born infants at term equivalent age. Neuroimage Clin. 2018;18:51-59.
[3] Young JM, Vandewouw MM, Mossad SI, et al. White matter microstructural differences identified using multi-shell diffusion imaging in six-year-old children born very preterm. Neuroimage Clin. 2019;23:101855.
[4] Dhollander T, Connelly A. A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+b=0) diffusion MRI data. 24th International Society of Magnetic Resonance in Medicine; Singapore, 2016.
[5] Khan W, Egorova N, Khlif MS, et al. Three-tissue compositional analysis reveals in-vivo microstructural heterogeneity of white matter hyperintensities following stroke. Neuroimage. 2020;218:116869.
[6] Mito R, Dhollander T, Xia Y, et al. In vivo microstructural heterogeneity of white matter lesions in healthy elderly and Alzheimer's disease participants using tissue compositional analysis of diffusion MRI data. NeuroImage: Clinical. 2020;28:102479.
[7] Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137.
[8] Jenkinson M, Beckmann CF, Behrens TE, et al. Fsl. Neuroimage. 2012;62(2):782–790.
[9] Kellner E, Dhital B, Kiselev VG, et al. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76(5):1574-1581.
[10] Andersson JLR, Graham MS, Drobnjak I, et al. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage. 2017;152:450-466.
[11] Andersson JLR, Graham MS, Zsoldos E, et al. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556-572.
[12] Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078.
[13] Schilling KG, Blaber J, Huo Y, et al. Synthesized b0 for diffusion distortion correction (Synb0-DisCo). Magn Reson Imaging. 2019;64:62-70.
[14] Dhollander T, Mito R, Raffelt D, et al. Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. 27th International Society of Magnetic Resonance in Medicine; Montréal, QC, Canada, 2019.
[15] Raffelt D, Tournier JD, Fripp J, et al. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage. 2011;56(3):1171-1180.
[16] Newman BT, Dhollander T, Reynier KA, et al. Test–retest reliability and long-term stability of three-tissue constrained spherical deconvolution methods for analyzing diffusion MRI data. Magnetic Resonance in Medicine. 2020;84(4):2161-2173.
[17] Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98.
[18] Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol. 2013;34(11):2208-2214.
[19] Ball G, Srinivasan L, Aljabar P, et al. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A. 2013;110(23):9541-9546.
Figures