2391
Decoupling of global brain activity and cerebrospinal fluid (CSF) flow was evident in Parkinson’s cognitive decline
Feng Han1, Gregory L Brown2,3, Yalin Zhu1, Aaron Belkin-Rosen1, Mechelle M Lewis3,4, Guangwei Du3, Yameng Gu1, Paul J Eslinger3,5, Richard B Mailman3,4, Xuemei Huang3,4,5,6,7,8, and Xiao Liu1,8
1Department of Biomedical Engineering, The Pennsylvania State University, State College, PA, United States, 2Department of Engineering Science and Mechanics, The Pennsylvania State University, State College, PA, United States, 3Department of Neurology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 4Department of Pharmacology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 5Department of Radiology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 6Department of Neurosurgery, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 7Department of Kinesiology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 8Institute for Computational and Data Sciences, The Pennsylvania State University, State College, PA, United States
1Department of Biomedical Engineering, The Pennsylvania State University, State College, PA, United States, 2Department of Engineering Science and Mechanics, The Pennsylvania State University, State College, PA, United States, 3Department of Neurology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 4Department of Pharmacology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 5Department of Radiology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 6Department of Neurosurgery, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 7Department of Kinesiology, Pennsylvania State University Milton S. Hershey Medical Center, Hershey, PA, United States, 8Institute for Computational and Data Sciences, The Pennsylvania State University, State College, PA, United States
Synopsis
Deposition and spreading of misfolded proteins have been linked to cognitive dysfunction in Parkinson’s disease (PD). The glymphatic system responsible for removing brain wastes thus may play a role in cognitive impairment in PD. This hypothesis is however difficult to test in clinical populations due to the lack of non-invasive measurements of glymphatic function. Low-frequency (< 0.1 Hz) resting-state fMRI (rsfMRI) signal was recently linked to glymphatic function primarily based on its sleep-dependent coupling with CSF flows. This study found early evidence that the coupling of global rsfMRI and CSF signals is indeed related to cognitive decline in PD.
Introduction
The pathology underlying Parkinson’s cognitive decline may involve the aggregation and spreading of α-synuclein (α-syn) to extra-nigral subcortical and cortical regions1,2. In the central nervous system, the glymphatic system is in charge of the clearance of toxic proteins, such as α-syn3,4, amyloid-β5,6, and tau7, through CSF flow, which was believed to be driven by arterial pulsation8 and respiration9. The glymphatic clearance is much stronger during sleep than awake, and this enhancement could be brought up by reduced tissue resistance due to increased interstitial space10. This hypothesis was supported by an increase of brain pulsations at the cardiac and respiratory frequencies, as measured by a fast fMRI technique11. The same study, however, found the rsfMRI signals of much lower frequency (< 0.1Hz) increased to a much larger extent during sleep than the fast pulsations11. Consistent with this observation, the global rsfMRI BOLD signal was found to be coupled with strong CSF flow at a timescale of >10 seconds during sleep12, and the vasomotor waves of a similar timescale were also linked to amyloid-β clearance13. Based on all these findings, we here tested the hypothesis that the coupling between the global BOLD signal and CSF flow is closely linked to the glymphatic function and thus cognitive dysfunction in PD patients.Methods
We included 60 PD and 58 control subjects from whom we collected resting-state fMRI data and measured Montreal Cognitive Assessment (MoCA) scores. We extracted CSF signals from the bottom slice of fMRI imaging volume, which is near the bottom of the cerebellum, to maximize sensitivity to the CSF inflow effect. The global BOLD signal was obtained by averaging rsfMRI signals from the gray-matter regions14. We calculated the cross-correlation function between the global BOLD (also its temporal derivative) and CSF signals, and then quantify the global BOLD-CSF (gBOLD-CSF) coupling using the value at the 4-sec lag, where we saw the largest (negative) peak. The age- and gender-adjusted gBOLD-CSF coupling metric was then compared between three groups: non-PD controls, PD subjects without mild cognitive impairment (MCI)(MoCA ≥ 26), and PD with MCI (MoCA < 26). This coupling index was then directly correlated with MoCA score, Unified Parkinson's Disease Rating Scale (UPDRS) score, and the structural markers for early-stage AD, including hippocampal volume and entorhinal cortex (ERC) thickness. These correlational analyses were conducted across all subjects or separately for the PD and control groups. We also re-examined the association between the gBOLD-CSF coupling and MoCA with removing some MoCA outliers and also controlling for disease duration, head motion quantified with mean framewise displacement (FD)15, and arousal level estimated by an fMRI-based drowsiness index15.Results
The cross-correlation function between gBOLD and CSF signals displayed a positive peak at the lag of -4 seconds (0.28; p < 0.0001, permutation test16) whereas a negative one at +4 seconds (-0.28; p < 0.0001). The negative derivative of the global BOLD showed a strong zero-lag positive correlation (0.37; p < 0.0001) with the CSF signals. Both patterns are consistent with those observed in the previous study12, confirming the strong gBOLD-CSF coupling in this PD dataset (Fig. 1). The gBOLD-CSF coupling, quantified by BOLD-CSF correlation at the 4-sec lag, appeared to be weaker (less negative) in older subjects (p =0.0005). After controlling for age and gender, the gBOLD-CSF coupling is significantly weaker (p < 0.01) in the PD-MCI group but has a similar strength in PD-non-MCI and control groups (p = 0.96). The weaker gBOLD-CSF coupling is significantly associated with a lower MoCA score across all the subjects (Spearman’s ρ = -0.25, p = 0.007). This association is stronger across PD subjects (ρ = -0.36, p = 0.005) but not significant for the controls (ρ = -0.06, p = 0.65) (Fig. 2). In contrast, the gBOLD-CSF coupling is not correlated (p > 0.27) with three UPDRS scores across PD subjects. The association between gBOLD-CSF coupling and MoCA remained significant (p < 0.05) after removing two MoCA outliers or controlling for disease duration, head motion (mean FD), and drowsiness index calculated based on resting-state fMRI (Fig. 3). Across PD subjects, the weaker gBOLD-CSF coupling was also associated with thinner (ρ = -0.36, p = 0.012) right ERC. Similar associations were also present but non-significant for the left ERC thickness and bilateral hippocampal volume (Fig. 4).Discussions
The global BOLD signal is strongly coupled with CSF changes in the PD dataset. This gBOLD-CSF coupling is significantly reduced in PD patients showing cognitive impairment compared to both controls and Parkinson’s patients without cognitive dysfunction. Moreover, the gBOLD-CSF coupling strength was correlated with cognitive scores but only in Parkinson’s patients and not controls, suggesting it is linked to Parkinson-related processes. The gBOLD-CSF coupling was not correlated with MDS-UPDRS motor or non-motor scores, suggesting it is specifically related to a cognitive component of Parkinson’s. The reduced gBOLD-CSF coupling also was associated with a thinner ERC that has been observed in early-stage Alzheimer’s17, suggesting a possible contribution from Alzheimer’s pathology in PD-MCI.Conclusion
These findings suggest that the gBOLD-CSF coupling is a potential noninvasive marker for the glymphatic function that may be related to dementia pathologies.Acknowledgements
We thank Jiaxuan Diao for preparing some figures and reviewing the abstract. This work was supported by the National Institutes of Health (NIH) Pathway to Independence Award (K99/R00 5R00NS092996-03), the Brain Initiative award (1RF1MH123247-01), and the NIH R01 award (1R01NS113889-01A1). This work was also supported by the National Institute of Neurological Disorders and Stroke Parkinson’s Disease Biomarker Program (NS06722 and NS112008 to X.H), the National Institute of Aging (AG067651 to G.B), the Hershey Medical Center General Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127), the PA Department of Health Tobacco CURE Funds, and the Penn State Translational Brain Research Center.References
- Hurtig, H. I. et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology (2000). doi:10.1212/WNL.54.10.19162.
- Mattila, P. M., Rinne, J. O., Helenius, H., Dickson, D. W. & Röyttä, M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. (2000). doi:10.1007/s0040199001683.
- Valdinocci, D. et al. Extracellular interactions of Alpha-synuclein in multiple system atrophy. International Journal of Molecular Sciences (2018). doi:10.3390/ijms191241294.
- Sundaram, S. et al. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neuroscience and Biobehavioral Reviews (2019). doi:10.1016/j.neubiorev.2019.05.0165.
- Tarasoff-Conway, J. M. et al. Clearance systems in the brain - Implications for Alzheimer disease. Nature Reviews Neurology (2015). doi:10.1038/nrneurol.2015.1196.
- Yulug, B., Hanoglu, L. & Kilic, E. Does sleep disturbance affect the amyloid clearance mechanisms in Alzheimer’s disease? Psychiatry and Clinical Neurosciences (2017). doi:10.1111/pcn.125397.
- Holth, J. K. et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science (80-. ). (2019). doi:10.1126/science.aav25468.
- Mestre, H. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. (2018). doi:10.1038/s41467-018-07318-39.
- Yamada, S. et al. Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS (2013). doi:10.1186/2045-8118-10-3610.
- Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science (80-. ). (2013). doi:10.1126/science.124122411.
- Helakari, H. et al. Sleep-specific changes in physiological brain pulsations. bioRxiv 2020.09.03.280479 (2020).12.
- Fultz, N. E. et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science (80-. ). (2019). doi:10.1126/science.aax544013.
- van Veluw, S. J. et al. Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain. Neuron (2020). doi:10.1016/j.neuron.2019.10.03314.
- Power, J. D. et al. Ridding fMRI data of motion-related influences: Removal of signals with distinct spatial and physical bases in multiecho data. Proc. Natl. Acad. Sci. U. S. A. (2018). doi:10.1073/pnas.172098511515.
- Gu, Y., Han, F., Sainburg, L. E. & Liu, X. Transient Arousal Modulations Contribute to Resting-State Functional Connectivity Changes Associated with Head Motion Parameters. Cereb. Cortex (2020). doi:10.1093/cercor/bhaa09616.
- Han, F. et al. The coupling of global brain activity and cerebrospinal fluid inflow is correlated with Alzheimer’s disease related pathology. bioRxiv 2020.06.04.134726 (2020). doi:10.1101/2020.06.04.13472617.
- Long, X., Zhang, L., Liao, W., Jiang, C. & Qiu, B. Distinct laterality alterations distinguish mild cognitive impairment and Alzheimer’s disease from healthy aging: Statistical parametric mapping with high resolution MRI. Hum. Brain Mapp. (2013). doi:10.1002/hbm.2215718.
- Smith, S. M. et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage (2002). doi:10.1006/nimg.2002.1040
Figures
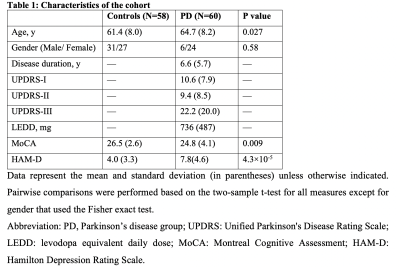
Table 1
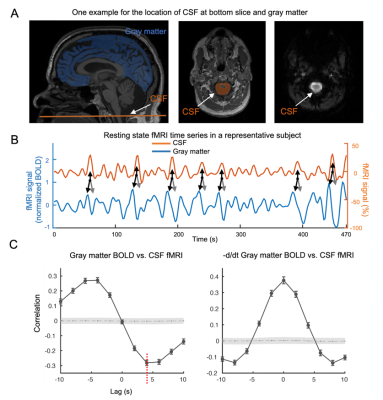
Fig. 1. Global BOLD is coupled with CSF changes. (A) left: global BOLD was averaged across gray matter; middle&right: CSF region at bottom fMRI slice. (B) Coupled changes of global BOLD and CSF signal from an example (indicated with arrows). (C) upper: averaged global BOLD-CSF cross-correlation across 118 subjects; lower: the one for the negative derivative of global BOLD and CSF signal. Shade: 95% confidence interval calculated with shuffled signals16. Error bar: standard error of the mean (SEM).
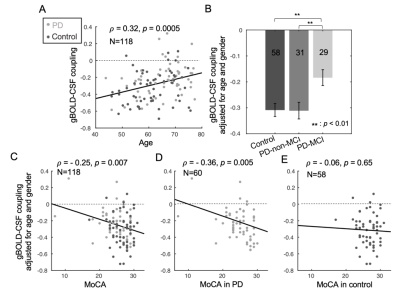
Fig. 2. The associations of gBOLD-CSF coupling to age, disease condition, MoCA, and UPDRS. (A) The strength of the gBOLD-CSF coupling (global BOLD-CSF correlation at +4-sec lag) decreased with age (Spearman’s ρ = 0.32, p = 0.0005). Age- and gender-adjusted gBOLD-CSF coupling is significant weaker in PD-MCI group (B) and significantly correlated with MoCA scores across all the subjects (C), within the PD group (D), but not across controls (E).
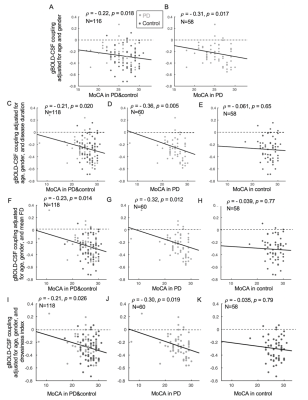
Fig. 3. The associations between gBOLD-CSF coupling and MoCA remained significant in the entire group and PD subjects but not controls, when removing two extreme low MoCA samples (MoCA < 15) (A-B) or controlling for the disease duration (C-E), head motion (F-H), and fMRI-based estimates of drowsiness level15 (I-K).
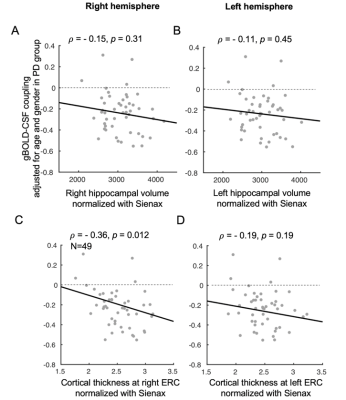
Fig. 4. Associations between gBOLD-CSF coupling and structural measures of the hippocampus and entorhinal cortex in PD patients. Correlation analyses suggested the gBOLD-CSF coupling significantly decreased with the normalized right ERC thickness increasing in PD subjects, while the same trend can be found when relating the gBOLD-CSF coupling to left ERC thickness and bilateral hippocampal volumes (all structural measures were normalized with the Sienax index quantifying brain tissue volume18).