2388
Real-time temperature correction of the relaxation parameters in in situ post-mortem neuro MRI1Institute of Forensic Medicine, Department of Biomedical Engineering, Basel, Switzerland, 2Institute of Forensic Medicine, Health Department Basel-Stadt, Basel, Switzerland
Synopsis
The temperature sensitivity of the relaxation parameters represents a major issue in post-mortem MRI due to the passive cooling of the deceased. This study proposes a real-time non-invasive temperature correction method of MR neuroimaging parameters based on an MRI compatible forehead temperature probe. The observed significant linear relations between the forehead temperature and the relaxation times indicate that this real-time non-invasive temperature correction method is suitable for in situ post mortem MR neuroimaging.
Introduction
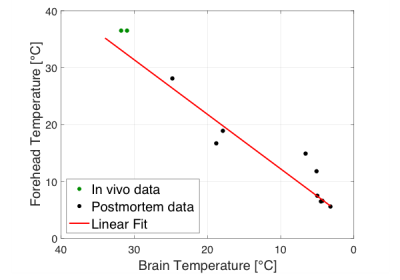
The temperature dependence of the magnetic resonance imaging parameters T1, T2, and T2* represents a major issue in post-mortem MRI due to the passive cooling of the deceased.1,2 A few journal articles were published on this topic 3,6, in which the MRI parameters were corrected for the body core temperature measured prior to the MRI scan. However, during the MRI measurement, the body temperature increases due to radio frequency pulses.7 This issue was only considered in one study, in which a real-time body core measurement was conducted during the scan.8 Moreover, it was shown that after death the core temperature decreases at a slower cooling rate than the brain temperature, questioning the temperature correction of MR neuroimaging based on the core temperature.9,10 In contrary, the forehead and the brain temperature revealed a linear correlation (see figure 1). As compared to the brain temperature, the forehead temperature can be assessed non-invasively and in real-time. Thus, in this study the effect of the forehead temperature on the brain’s relaxation parameters was investigated to correct the parameters in real-time during the MRI scan and to therefore lay the foundation for future projects on post-mortem validation of in vivo neuro MRI techniques.Methods
12 deceased underwent in situ post-mortem MRI brain examination on a 3T scanner using a defined protocol [T1: IR-SE, TE/TR: 12/7060ms, T2: multi-echo SE, TE/TR: 9.8-117.6/5720ms, T2*: GRE, TE/TR: 5.79-50.94/68ms]. In addition to the post-mortem examinations, two healthy subjects volunteered for the similar MRI protocol. The forehead temperature was measured in situ using an MR compatible temperature probe. The temperature effect was computed by fitting a linear model to the MRI parameters of white matter (WM) and gray matter (GM) brain tissue and the mean forehead temperature of the corresponding MRI sequence.Results
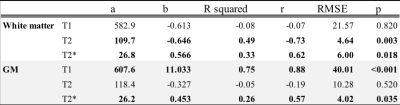
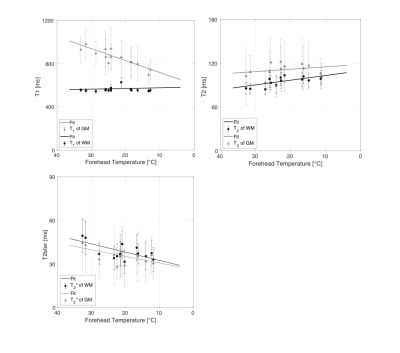
T1 of gray matter prolongs significantly for an increasing forehead temperature, while T1 of white matter reveals no temperature dependence. T2 increases significantly for a decreasing forehead temperature in WM, while in gray matter no significant temperature dependence could be observed. T2* revealed significant temperature dependence in both tissue types (see table 1 and figure 2).Discussion
The detected linear relations between the forehead temperature assessed in real time and the MR neuroimaging parameters indicate that the forehead temperature is appropriate to correct the MR neuroimaging parameters. This was expected regarding the relation between the forehead and brain temperature. T1 of WM might not be temperature dependent due to the limited molecular mobility in the intracellular cytoplasm of the myelinated axons impacting the longitudinal relaxation rate. The data size was insufficient to retrieve significant correlations between T2 and GM.Conclusion
The results indicate that the forehead temperature is suitable to correct MR neuroimaging parameters. In contrast to the core or brain temperature assessed prior to the scan, this allows correcting MR neuroimaging parameters with a non-invasive and a real-time temperature measurement.Acknowledgements
No acknowledgement found.References
1. Nelson, T.R. and S.M. Tung, Temperature dependence of proton relaxation times in vitro. Magn Reson Imaging, 1987. 5(3): p. 189-99.
2. Birkl, C., et al., Temperature-induced changes of magnetic resonance relaxation times in the human brain: a postmortem study. Magn Reson Med, 2014. 71(4): p. 1575-80.
3. Ruder, T.D., et al., The influence of body temperature on image contrast in post mortem MRI. Eur J Radiol, 2012. 81(6): p. 1366-70.
4. Busch, J.R., et al., Post-mortem MRI-based volumetry of the hippocampus in forensic cases of decedents with severe mental illness. Forensic Sci Med Pathol, 2019. 15(2): p. 213-217.
5. Tashiro, K., et al., Cerebral Relaxation Times from Postmortem MR Imaging of Adults. Magnetic Resonance in Medical Sciences, 2015. 14(1): p. 51-56.
6. Zech, W.D., et al., Post-mortem 1.5T MR quantification of regular anatomical brain structures. Int J Legal Med, 2016. 130(4): p. 1071-1080.
7. Kim M S. Investigation of Factors Affecting Body Temperature Changes During Routine Clinical Head Magnetic Resonance Imaging, Iran J Radiol. 2016 ; 13(4):e34016. doi: 10.5812/iranjradiol.34016.
8. van den Brink, J.S., Thermal Effects Associated with RF Exposures in Diagnostic MRI: Overview of Existing and Emerging Concepts of Protection. Concepts in Magnetic Resonance Part B, 2019. 2019: p. 9618680.
9. Bartgis, C., et al., Determination of time of death in forensic science via a 3-D whole body heat transfer model. J Therm Biol, 2016. 62(Pt B): p. 109-115.
10. Gulyas, B., et al., Continuous monitoring of post mortem temperature changes in the human brain. Neurochem Res, 2006. 31(2): p. 157-66.
Figures