2381
Real Time 4D flow MRI assessment of Low Frequency Oscillations in Large Intracranial Vessels at Rest and During Hypercapnia1University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Low frequency oscillations (LFOs) of cerebral vessels may be important indicators of aging and disease related changes to cerebral autoregulation and glymphatic clearance. Assessing LFOs during a vasodilatory stimulus, such as hypercapnia, may reveal age-associated changes not apparent at rest. This study utilized 3D radial sampling and local low-rank reconstruction of 4D flow MRI scans to achieve real time temporal resolution to assess LFOs. Preliminary results suggest that there were no hypercapnia or age-associated changes in LFOs in the large intracranial arteries; however, there were both age and hypercapnia effects on LFOs in the venous circulation.
Introduction
Analysis of oscillations in the low frequency spectrum (LFOs) in cerebral vessels may be a useful tool to assess cerebrovascular function. Impairments in LFOs have been implicated in carotid artery disease, 1 ischemic stroke, 1 Alzheimer’s disease 2 and aging 3 and may indicate reduced autoregulatory function and glymphatic clearance. 4 Commonly used methods to measure LFOs in the cerebral vessels include near-infrared spectroscopy (NIRS) and blood-oxygen level derived (BOLD) functional magnetic resonance imaging (fMRI). However, both techniques are limited by their indirect blood flow measurement derived from oxygenated and deoxygenated hemoglobin. Recently, 4D flow MRI has been utilized to assess LFOs in the large intracranial vessels. 2 This technique applies both 3D radial sampling and a local low-rank reconstruction approach to feasibly achieve real time temporal resolution. Using this approach, LFOs have been assessed at rest; however, aging is associated with a reduction in cerebral vasodilatory capacity, 5 and age-related changes in LFOs may be more apparent during hypercapnia. The purpose of this study was to evaluate LFOs in both the arterial and venous cerebral vasculature at rest and, for the first time, during hypercapnia using 4D flow MRI. Our exploratory analysis evaluated the effect of primary aging on LFOs parameters.Methods
This study included 10 healthy participants with low vascular risk (5 young adults, age=24±1, men=3, women=2; 5 middle-aged/older adults, age=62±2, men=3, women=2). Participants were fitted with an MRI compatible facemask with a one-way value to prevent re-breathing. They were instrumented with a pulse oximeter to measure heart rate and oxygen saturation (SPO2), a nasal cannula to measure end-tidal carbon dioxide (ETCO2), and a blood pressure cuff to measure mean arterial pressure (MAP). MR imaging was performed with a 3T clinical scanner (MR750, GE Healthcare) with a 32-channel head coil (Nova Medical Head Coil). 4D flow MRI data were acquired using a 3D radially undersampled sequence. 6 The scan parameters were as follows: velocity encoding (Venc) = 80 cm/s, FOV = 220 mm, acquired spatial resolution = 0.7 mm isotropic, TR = 7.8 ms, TE = 2.7 ms, flip angle = 8°, bandwidth = 83.3 kHz, 14,000 projection angles and scan time ∼7 min. The first scan was acquired while participants were breathing medical grade normoxic, normocapnic air (baseline). The second scan was acquired while participants were breathing a hypercapnic gas mixture that contained 6% CO2, 21% O2 and balanced nitrogen (hypercapnia). The flow encoded images from the 4D flow MRI scans were reconstructed to 100 frames using GPU accelerated iterative SENSE with a local low-rank constraint. 7–9 Reconstructions were performed with 8x8x8 blocks and a manually tuned regularization parameter. The reconstructed spatial resolution was 1.38mm isotropic and the temporal resolution was approximately 4.3 seconds. Vessel segmentation and LFO analysis was performed offline in MATLAB (Mathworks). 10 Flow parameters were extracted from both the left and right internal carotid arteries (ICAs) (between the cervical and petrous segments) as well as the posterior, inferior portion of the superior sagittal sinus (SSS). Flow ranges, standard deviations and standard deviation normalized to brain volume were calculated for each vessel. In addition, a Fourier transform was performed on the flow time series. The power spectral density (PSD) was calculated between 0.002 and 0.10 Hz. LFO parameters were compared between baseline and hypercapnia using a 2-tailed paired t-test. The effect of aging on LFO parameters was assessed using a 2-tailed unpaired t-test, alpha <0.05.Results
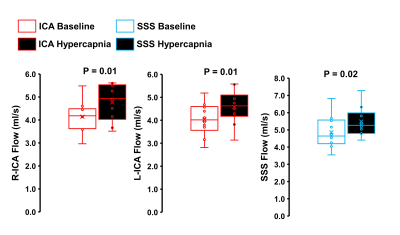
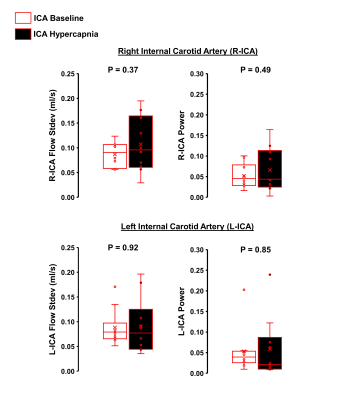
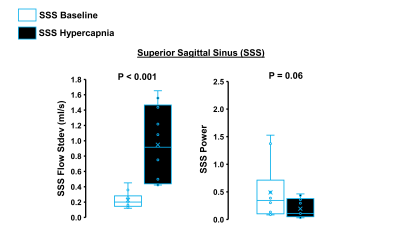
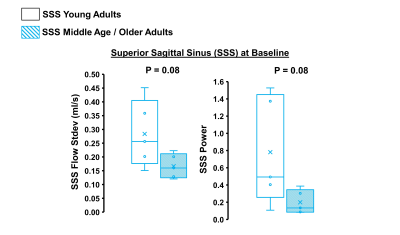
Hypercapnia increased flow in the right ICA, left ICA, and the SSS as expected (Figure 1, p<0.05 for all). In the ICAs, there were no effects of hypercapnia on LFO parameters including flow range, flow standard deviation, and low-frequency power (Figure 2, p>0.05 for all). In the SSS, hypercapnia was associated with increased flow range (p<0.01), increased flow standard deviation (p<0.001), and a trend for decreased average low-frequency power (p=0.06; Figure 3). Results were similar when flow standard deviation was normalized to brain volume. There were no age-related differences in LFO parameters in the right and left ICAs at baseline or hypercapnia (p>0.05). However, there was a trend for smaller flow range (p=0.06), smaller flow standard deviation (p=0.08), and lower low-frequency power (p=0.08) in the SSS at baseline in middle-aged/older adults compared with young adults (Figure 4). There were no age-related differences in LFO parameters of the SSS during hypercapnia (p>0.05 for all).Discussion and Conclusions
We observed no change in LFOs from baseline to hypercapnia in the arterial system (ICAs). However, hypercapnia caused changes in LFO parameters in the venous system (SSS) including an increase in flow variations and a trend of reduced LFO power. Because flow in the SSS is sensitive to changes in MAP and respiration, our results suggest that LFO changes during hypercapnia may be related to systemic, rather than cerebral, factors. We also observed a trend for an age-related reduction in LFO parameters in the SSS at baseline. This suggests that primary aging may be associated with changes in LFOs in the venous circulation. Future work is needed to determine the mechanisms by which hypercapnia may induce changes in 4D flow based LFOs.Acknowledgements
We acknowledge funding support from the Wisconsin Alumni Research Foundation (JNB), NIH grants HL118154 (JNB), NS117746 (JNB), and Alzheimer's Association grant AARFD-20-678095s (LARR).References
1. Schytz HW, Hansson A, Phillip D, Selb J, Boas DA, Iversen HK et al. Spontaneous Low-Frequency Oscillations in Cerebral Vessels: Applications in Carotid Artery Disease and Ischemic Stroke. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2010; 19: 465–474.
2. Rivera-Rivera LA, Cody KA, Rutkowski D, Cary P, Eisenmenger L, Rowley HA et al. Intracranial vascular flow oscillations in Alzheimer’s disease from 4D flow MRI. NeuroImage Clin 2020; 28. doi:10.1016/j.nicl.2020.102379.
3. Schroeter ML, Schmiedel O, von Cramon DY. Spontaneous Low-Frequency Oscillations Decline in the Aging Brain. J Cereb Blood Flow Metab 2004; 24: 1183–1191.
4. Goodman JR, Iliff JJ. Vasomotor influences on glymphatic-lymphatic coupling and solute trafficking in the central nervous system. J Cereb Blood Flow Metab 2020; 40: 1724–1734.
5. Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O et al. Age-Related Reductions in Cerebrovascular Reactivity Using 4D Flow MRI. Front Aging Neurosci 2019; 11. doi:10.3389/fnagi.2019.00281.
6. Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008; 60: 1329–1336.
7. Jimenez JE, Strigel RM, Johnson KM, Henze Bancroft LC, Reeder SB, Block WF. Feasibility of high spatiotemporal resolution for an abbreviated 3D radial breast MRI protocol. Magn Reson Med 2018; 80: 1452–1466.
8. Ying L, Sheng J. Joint image reconstruction and sensitivity estimation in SENSE (JSENSE). Magn Reson Med 2007; 57: 1196–1202.
9. Ong F, Lustig M. SigPy: A Python Package for High Performance Iterative Reconstruction. 27th Annual ISMRM Meeting Montreal, Canada; : 4819.
10. Schrauben E, Wåhlin A, Ambarki K, Spaak E, Malm J, Wieben O et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging 2015; 42: 1458–1464.
Figures