2380
Improving Automatic Cerebral Microbleed Detection Using Algorithmic Methods in Multi-Echo STAGE Data1Magnetic Resonance Innovations, Inc., Bingham Farms, MI, United States, 2Wayne State University, Detroit, MI, United States, 3Tianjin First Central Hospital, Tianjin, China
Synopsis
Automatic cerebral microbleed detection is attainable with our two step model for many disease states. We attributed previously shown lower performance in STAGE data to veins and edges, including some in the basal ganglia. We improved our existing pipeline for this detection by adding a false positive correction step to our pipeline using previously tested and new data. The results were improved overall, including on previously tested STAGE data, new STAGE data and our previously tested single echo data (multiple diseases). This makes our pipeline a viable and versatile real time automatic microbleed detection procedure.
Introduction
Cerebral microbleeds (CMBs) are small foci of blood products found in patients affected by a multitude of conditions, including Alzheimer's disease, stroke, and traumatic brain injury1-2. While the location of CMBs has been associated with etiology, the number of CMBs could predict future cognitive or vascular problems3-4. Susceptibility weighted imaging (SWI) proves to be a powerful tool by which to detect CMBs. We previously reported on a two-stage CMB detection framework based on a 3D fast radial symmetry transform and a deep residual neural networks model using SWI and high-pass filtered phase images. Our findings included different clinical etiologies and were published in Liu et al. 20195 and reported in the 2020 ISMRM abstract #3565. That work achieved an overall sensitivity of 95.8% and 1.3 false positives (FPs) per case for combined etiologies and 93.5% sensitivity and 2.7 FPs on average per case for stroke cases only. These performance metrics were measured on single echo SWI validation data, however, and we intend on reaching similar results using strategically acquired gradient echo (STAGE) data, on which the CMB detection performed less ideally. STAGE is a multi-scan multi-echo sequence that provides comprehensive brain imaging with minimal acquisition time6-7. It also includes a contrast agent-free MRAV created using a subtraction of an interleaved dephased and rephased scans8, which allows us to correct some of the false positives in the CMB detection.Methods
We organized a total of 97 cases, including 40 cases of previously collected and tested data (cohort I), and 57 new datasets (cohort II) from Tianjin First People Hospital in China. We used an enhanced edge detection method, a sequence derived vein mask, and a modified candidate detection to eliminate FPs without affecting sensitivity. Although our CMB detection has a built in BET brain extraction step, many areas around the air tissue interface continued to create false positives. We used a voxel based mathematical division of the magnitudes from two echo times in the same scan, forming a well-defined brain boundary (see figure 1A-1C). We also used STAGE MRAV to create a global brain mask of vessels overlaid on CMB detection findings (see figure 1D-1F). This works well for large or superficial veins, but leaves some small veins without enough contrast to be identified. Most importantly, this mask is highly selective against CMBs, so applying it does not reduce sensitivity values (see figures 2-3). In the candidate detection step of the pipeline, the data is registered to the MNI152 template, which we used to increase the threshold needed for the globus pallidus and the putamen. These structures commonly contained false positives. Since we had obtained the gold standard on some data from experienced reviewers previously, our analysis involved creating a similar gold standard for the new datasets and simply comparing the performance measurements previously reported as well as reporting the number of FPs found in each cohort.Results
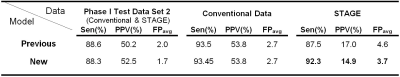
Combining the vein and edge masks with a modified candidate detection, we eliminated false positives that were initially included by the AI algorithm in the STAGE dataset (see tables 1-2). The sensitivity from previous work was 87.5% and it reached 92.3% for the current work, while the average FPs were reduced from 4.6 to 3.7. We compared the performance of the detection on two test cohorts, including the number of FPs on the same STAGE datasets showing 20 fewer false positives (in 40 cases) for the current work (see figure 3C). We also tested the latest pipeline on a cohort of new STAGE datasets and noted similar improvement in the number of FPs (see figure 3D).Discussion
The methods we added in this work have successfully eliminated many false positives without affecting the sensitivity of the overall pipeline. We learned from previous work that vessels and edges are the most common false positives we have found for all data. The algorithmic methods presented in this work take advantage of the STAGE imaging sequence and build on our previous work in automatic CMB detection. Although these results are impressive for a fast sequence such as STAGE, we plan to incorporate the MRAV images as new channels into the AI model, possibly yielding superior detection performance. We are in a position to include the contrasts mentioned in this work and others, such as R2*, T1 map, SWI vesselness, or commutator filter9 as channels for the AI portion, where we can test the viability of such a model and the advantages compared to algorithmic approaches.Conclusion
In conclusion, adding a false positive correction step improved the automatic CMB detection performance on STAGE data. This algorithmic approach was effective at fine tuning some pitfalls in the existing pipeline, bringing automatic CMB detection one step closer to being a viable option as a real time processing method in the clinical world.Acknowledgements
Dr. Luo Yu
Shanghai Fourth Province People Hospital
Shanghai, China
Dr. Shuang Xia
Tianjin First Center Hospital
Tianjin, China
References
1- Greenberg, S.M., Vernooij, M.W., Cordonnier, C., Viswanathan, A., Al-Shahi Salman, R.,Warach, S., et al., 2009. Cerebral microbleeds: a guide to detection andinterpretation. Lancet Neurol. 8 (2), 165–174.
2- Yates, P.A., Villemagne, V.L., Ellis, K.A., Desmond, P.M., Masters, C.L., Rowe, C.C., 2014.Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations.Front. Neurol. 4, 205.
3- Fan, Yu Hua, Zhang, Lei, Lam Wynnie, W.M., Mok Vincent, C.T., Wong, Ka Sing, 2003.Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhagesamong patients with acute ischemic stroke. Stroke 34 (10), 2459–2462.
4- Poels, M.M.F., Ikram, M.A., van der Lugt, A., Hofman, A., Niessen, W.J., Krestin, G.P.,et al., 2012. Cerebral microbleeds are associated with worse cognitive function: theRotterdam Scan Study. Neurology 78 (5), 326–333.
5- Liu, S., Utriainen, D., Chai, C., Chen, Y., Wang L., Sethi, S. K., Xia, S., Haacke, E. M., 2019. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. Neuroimage 198, 271–282.
6- Chen Y, Liu S, Wang Y, Kang Y, Haacke EM. STrategically Acquired Gradient Echo (STAGE) imaging, part I: Creating enhanced T1 contrast and standardized susceptibility weighted imaging and quantitative susceptibility mapping. Magn Reson Imaging. 2018 Feb;46:130-139. doi: 10.1016/j.mri.2017.10.005. Epub 2017 Oct 19. PMID: 29056394.
7- Wang Y, Chen Y, Wu D, Wang Y, Sethi SK, Yang G, Xie H, Xia S, Haacke EM. STrategically Acquired Gradient Echo (STAGE) imaging, part II: Correcting for RF inhomogeneities in estimating T1 and proton density. Magn Reson Imaging. 2018 Feb;46:140-150. doi: 10.1016/j.mri.2017.10.006. Epub 2017 Oct 20. PMID: 29061370.
8- EM. Haacke, Y. Chen, D. Utriainen, B. Wu, Y. Wang, S. Xia, et al.. Strategically Acquired Gradient Echo (STAGE) imaging, part III: Technical Advances and Clinical Applications of A Rapid Multi-Contrast Multi-Parametric Brain Imaging Method. Magnetic Resonance Imaging, 2020; 65:15-26. PMID: 31629075
9- Lai S, Reichenbach JR, Haacke EM. Commutator filter: a novel technique for the identification of structures producing significant susceptibility inhomogeneities and its application to functional MRI. Magn Reson Med. 1996 Nov;36(5):781-7. doi: 10.1002/mrm.1910360518. PMID: 8916030.
Figures