2372
Decoupling between global brain activity and cerebrospinal fluid flow is associated with Alzheimer's disease pathologies1Department of Biomedical Engineering, The Pennsylvania State University, State College, PA, United States, 2Department of Sociology & Criminology, The Pennsylvania State University, State College, PA, United States, 3Population Research Institute, The Pennsylvania State University, State College, PA, United States, 4Department of Biobehavioral Health, The Pennsylvania State University, State College, PA, United States, 5Institute for Computational and Data Sciences, The Pennsylvania State University, State College, PA, United States
Synopsis
Glymphatic system responsible for brain waste clearance may play an important role in the development of Alzheimer's disease (AD). However, this possibility has not been sufficiently studied in AD patients due to a lack of non-invasive tools for gauging glymphatic function. Low-frequency (<0.1 Hz) fMRI signals have been recently linked to glymphatic function, and its global signal was found to be coupled with CSF flow known to be essential for glymphatic clearance. Here, we used the coupling of global BOLD signal and CSF to quantify glymphatic function and found this BOLD-CSF coupling metric is significantly correlated with various AD pathologies.
Introduction
Alzheimer’s disease (AD) pathogenesis is widely believed to be driven by the aggregation of toxic proteins, particularly amyloid-β (Aβ) and tau1,2. The glymphatic system plays an important role in clearing out these toxic proteins from the extracellular interstitial space3,4. The glymphatic clearance is much stronger during sleep3, consistent with the diurnal fluctuation of interstitial Aβ5 and tau6. Arterial pulsation7 and respiration8 have been regarded as the major driving forces for glymphatic CSF flows. Although these physiological functions reduce during sleep9–12, brain pulsations of the same frequency ranges, measured by a fast fMRI method, indeed increased13 presumably due to lower tissue resistance with decreased interstitial space3. However, low-frequency (<0.1 Hz) resting-state fMRI (rsfMRI) signals were found to increase to a much larger extent than these brain pulsations of cardiac and respiratory frequencies during sleep13. Consistent with this, the global mean rsfMRI signal was found to be coupled by strong CSF flows expected to be essential for glymphatic clearance14. We hypothesize that this coupling process of global BOLD and CSF signals is tightly linked to glymphatic function, and thus AD pathologies. To test this hypothesis, we examined multimodal data from the Alzheimer's Disease Neuroimaging Initiative (ADNI)15 and investigated the association between the global BOLD-CSF coupling and various AD-related measurements.Methods
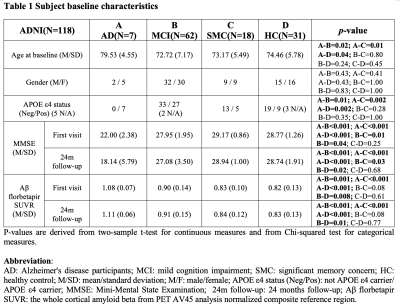
We used 158 sessions of imaging and behavioral data from 118 subjects in the ADNI project, which was selected based on the availability of rsfMRI, Aβ measurement using florbetapir positron emission tomography, and behavioral measures through Mini-Mental State Examination (MMSE). The CSF signal changes originate from MR inflow effect14, we extracted CSF signals from the bottom slice of fMRI acquisition to maximize this effect. The global BOLD signal was obtained by averaging signals from gray-matter regions16. The cross-correlation function was then computed between the global BOLD signal (also its temporal derivatives) and CSF signal. We then defined the global BOLD-CSF (gBOLD-CSF) coupling as their correlation at the negative peak of +3 sec time lag, and correlated this coupling metric to age, gender, disease condition (i.e., AD, mild cognitive impairment (MCI), significant memory concern, and control), APOE 𝛆4 allele number, baseline cortical Aβ and MMSE, and their changes in the following two years. A linear mixed model taking the subject as a random intercept17 was applied to account for the dependency data collected from the same subject (see detailed methods in our preprint paper18).Results
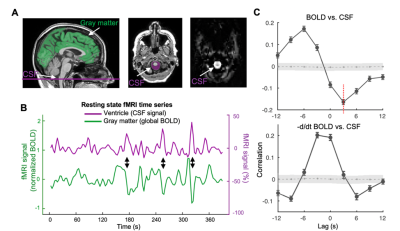
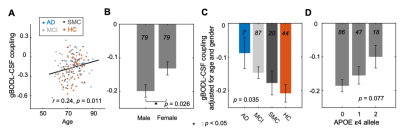
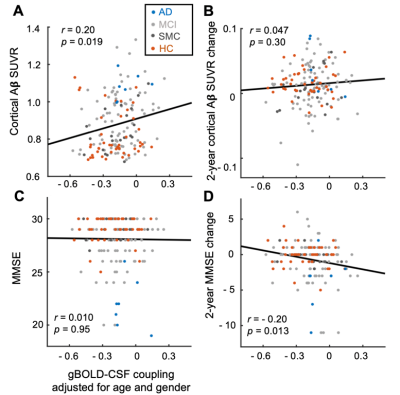
The mean BOLD-CSF cross-correlation function displayed a positive peak at -6-sec lag (0.17; p < 0.0001, permutation test18) whereas a negative one at the +3-sec lag (-0.17; p < 0.0001). The mean cross-correlation function between the negative global BOLD derivative and CSF signal showed a positive peak around the lag of -3 second (0.20; p < 0.0001) (Fig. 1). These patterns are consistent with those seen in the previous study14 and confirmed a strong coupling between the global BOLD and CSF fMRI from the ADNI subjects. The BOLD-CSF correlation at the 3-sec lag (i.e., negative peak) was used to quantify the strength of gBOLD-CSF coupling, which appeared weaker (less negative) in older (Spearman’s ρ = 0.24; p = 0.011), female (p = 0.026), more severe disease condition (p = 0.035), or AD-risk gene APOE 𝛆4 carrier (p = 0.077) (Fig. 2). More importantly, the subjects with weaker gBOLD-CSF coupling showed higher cortical Aβ accumulations (Spearman’s ρ = 0.20, p = 0.019) and larger reduction in cognitive MMSE score in the subsequent 2 years (ρ = -0.20, p = 0.013) (Fig. 3). The associations between the gBOLD-CSF coupling and Aβ accumulations and 2-year MMSE changes remained significant (p < 0.05) with controlling for the subjects’ arousal level as measured by the fluctuation amplitude of gBOLD signals and head motion as measured by mean framewise displacement (FD)19.Discussions
We demonstrated a strong coupling between the global BOLD signal and CSF flow using the resting-state fMRI data from an early-stage AD dataset. We found that the gBOLD-CSF coupling varied considerably across subjects and is significantly weaker in older subjects and females who have a higher risk of developing AD. The gradual weakening of this coupling metrics was also evident from the HC, to SMC, to MCI, and then to AD groups that showed increasing severity of AD-related symptoms. More importantly, the reduced gBOLD-CSF coupling was significantly associated with increased cortical Aβ level, and reduced MMSE score over the subsequent 2 years. All these results suggested a tight link between the gBOLD-CSF coupling and the AD pathology. It has been shown that the global BOLD signal was caused by a characteristic global brain activity representing transient arousal modulations20,21. This arousal-related neural modulation is coupled by slow (< 0.1Hz) but strong physiological modulations, particularly arterial pulsation and respiration16,19,22, which in turn may drive CSF flows and lead to the gBOLD-CSF coupling. Altogether, this coordinated neural and physiological process provides a sleep-dependent driving force for CSF flow essential for glymphatic clearance and could thus be linked to AD pathology.Conclusion
Our findings provide initial evidence linking the gBOLD-CSF coupling to AD pathologies, and also open the possibility of using this coupling metric as an surrogate marker for quantifying dynamic glymphatic function.Acknowledgements
We would like to thank Yalin Zhu and Abhiraj Saxena for processing data. We thank the ADNI for data collection and sharing through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
This work was supported by the National Institutes of Health Pathway to Independence Award (K99/R00 5R00NS092996-03), the Brain Initiative award (1RF1MH123247-01), and the NIH R01 award (1R01NS113889-01A1). Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering.
References
- Bloom, G. S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. (2014). doi:10.1001/jamaneurol.2013.58472.
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nature Reviews Neuroscience (2018). doi:10.1038/s41583-018-0067-33.
- Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science (80-. ). (2013). doi:10.1126/science.12412244.
- Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. (2012). doi:10.1126/scitranslmed.30037485.
- Kang, J. E. et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science (80-. ). (2009). doi:10.1126/science.11809626.
- Holth, J. K. et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science (80-. ). (2019). doi:10.1126/science.aav25467.
- Mestre, H. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. (2018). doi:10.1038/s41467-018-07318-38.
- Yamada, S. et al. Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS (2013). doi:10.1186/2045-8118-10-369.
- Douglas, N. J., White, D. P., Pickett, C. K., Weil, J. V. & Zwillich, C. W. Respiration during sleep in normal man. Thorax (1982). doi:10.1136/thx.37.11.84010.
- Baust, W. & Bohnert, B. The regulation of heart rate during sleep. Exp. Brain Res. (1969). doi:10.1007/BF0023544211.
- Boudreau, P., Yeh, W. H., Dumont, G. A. & Boivin, D. B. Circadian variation of heart rate variability across sleep stages. Sleep (2013). doi:10.5665/sleep.323012.
- Guazzi, M. & Zanchetti, A. Carotid sinus and aortic reflexes in the regulation of circulation during sleep. Science (80-. ). (1965). doi:10.1126/science.148.3668.39713.
- Helakari, H. et al. Sleep-specific changes in physiological brain pulsations. bioRxiv 2020.09.03.280479 (2020).14.
- Fultz, N. E. et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science (80-. ). (2019). doi:10.1126/science.aax544015.
- Weiner, M. W. et al. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimer’s and Dementia (2013). doi:10.1016/j.jalz.2013.05.176916.
- Power, J. D. et al. Ridding fMRI data of motion-related influences: Removal of signals with distinct spatial and physical bases in multiecho data. Proc. Natl. Acad. Sci. U. S. A. (2018). doi:10.1073/pnas.172098511517.
- Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. (2015). doi:10.18637/jss.v067.i0118.
- Han, F. et al. The coupling of global brain activity and cerebrospinal fluid inflow is correlated with Alzheimer’s disease related pathology. bioRxiv 2020.06.04.134726 (2020). doi:10.1101/2020.06.04.13472619.
- Gu, Y., Han, F., Sainburg, L. E. & Liu, X. Transient Arousal Modulations Contribute to Resting-State Functional Connectivity Changes Associated with Head Motion Parameters. Cereb. Cortex (2020). doi:10.1093/cercor/bhaa09620.
- Liu, X. et al. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. (2018). doi:10.1038/s41467-017-02815-321.
- Liu, X. et al. Arousal transitions in sleep, wakefulness, and anesthesia are characterized by an orderly sequence of cortical events. Neuroimage (2015). doi:10.1016/j.neuroimage.2015.04.00322.
- Özbay, P. S. et al. Contribution of systemic vascular effects to fMRI activity in white matter. Neuroimage (2018). doi:10.1016/j.neuroimage.2018.04.045
Figures