2369
Callosal Angle Biomarker for Normal Pressure Hydrocephalus Calculated for 4,980 T1-Weighted MR Exams1Radiology, Children's Hospital Los Angeles, Los Angeles, CA, United States, 2Rudi Schulte Research Insitute, Santa Barbara, CA, United States, 3Barrow Neurological Institute, Phenoix, AZ, United States, 4Children's Hospital Los Angeles, Los Angeles, CA, United States
Synopsis
Normal pressure hydrocephalus is a treatable dementia often misdiagnosed as Alzheimer’s Disease. Callosal angle measurement distinguish normal pressure hydrocephalus from other dementias but measurements are time-consuming and subjective. Therefore, we developed an algorithm for automatic callosal angle measurement and calculated callosal angles for 4,980 T1-weighted MRIs from databases of patients with purportedly healthy aging brains or Alzheimer’s. Based on the published guidelines, 1.8-4.3% of exams in these databases indicate patients likely have normal pressure hydrocephalus and thus a treatable form of dementia. Our automatic measurement of the callosal angle biomarker can rapidly and objectively screen patients for normal pressure hydrocephalus.
Introduction
We want to detect and treat normal pressure hydrocephalus (NPH) in elderly patients. Typical NPH symptoms include dementia, incontinence, and gait issues.1 These symptoms are nonspecific to NPH and are often misdiagnosed as Alzheimer’s or Parkinson’s disease. However, NPH can be treated effectively by shunting the ventricles. Thus reliably detecting NPH would prevent misdiagnosis of patients and create opportunities for successful treatment of their dementia.Measurement of the corpus callosal angle is a validated biomarker with NPH diagnostic accuracies of 93%, 77.8%, and 88.9%, for angles of 90°, 90.8°, and 100° as validated in studies of N=102, 90, and 318 patients, respectively.2-4 But manual measurement of callosal angle to rule out NPH is time-consuming and impractical in standard radiological practice. Thus, we developed an algorithm for automatic callosal angle measurement, validated it against an expert neuroradiologist, and screened two open-access datasets of elderly patients for abnormal callosal angles.
Methods
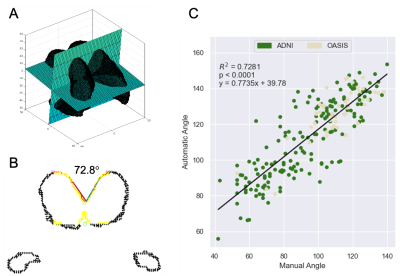
We selected 3,243 studies from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset5 and 1,737 studies from the Open Access Series of Imaging Studies (OASIS) dataset.6 ADNI neuroimaging was performed on 1.5 and 3T GE, Philips Medical Systems, and Siemens scanners; OASIS T1-weighted MRIs were acquired on 1.5 and 3T Siemens scanners.Automatic callosal angle measurements were performed using MATLAB (Mathworks, Natick, MD). All images were preprocessed using Freesurfer7 to align images to a standard orientation and extract the left and right lateral ventricles, the choroid plexus, and the third ventricle. Our automatic callosal angle algorithm (1) creates a reference axial plane through the centroid of the extracted ventricles and the anterior left and right lateral ventricles; (2) creates an oblique coronal plane perpendicular to the reference axial plane at the centroid; (3) fits a straight line through the ventricle walls; and (4) calculates the angle between these two lines.
Callosal angles (N=231) were manually acquired by a board-certified neuroradiologist (KK,15 years clinical experience); the method8 was: (1) identify a mid-sagittal slice; (2) create an axial reference plane through the anterior commissure and posterior commissure; (3) create a coronal reference plane perpendicular to the axial reference plane and at the level of the anterior commissure; (4) manually draw two straight lines through the ventricle walls; and (5) calculate the angle between these two lines.
Results
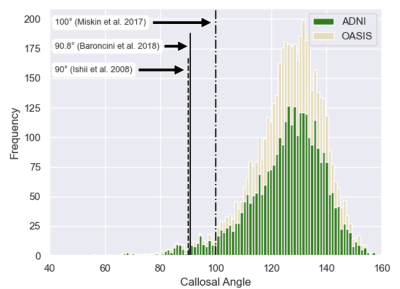
An example of the automatic continuous angle measurement for a narrow callosal angle is shown in Figure 1. Selection of the axial and coronal reference planes is indicated in Figure 1A. From the coronal reference plane, Figure 1B demonstrates the identification of points along the corpus callosum and fitting of lines to calculate the callosal angle. Figure 1C shows the linear regression of the manual versus automatic measurements. A histogram of the automatic callosal angle for the ADNI and OASIS datasets is presented in Figure 2. Using the proposed thresholds of 90°, 90.8°, and 100°,2-4 we found that 88, 93, and 215 of the 4,980 patients (1.8, 1.9, and 4.3%) are within the range for suspected NPH.Discussion
Correlation between the manual and automatic measurements of callosal angle indicates differences. However, the automated approach eliminates sources of intra- and inter-rater variation when selecting the reference planes and fitting the sliced callosal walls with a callosal angle. The automated landmarks were selected to be robust features that approximated the manual reference plans. Systematic differences between the approaches exist due to the different anatomical landmarks used by each approach. The presence of acute callosal angles was surprising given that the ADNI and OASIS datasets are not meant to contain patients with NPH. However, the finding corroborates premises that (1) the existing callosal angle NPH biomarker is not routinely used, and (2) that NPH cases may be misdiagnosed as Alzheimer’s Disease. Automatic callosal angle measurement can rapidly identify the cases requiring further screening, thus improving detection of patients with treatable dementia.Conclusion
Callosal angle measurements are an established tool to assess risk for normal pressure hydrocephalus. Our automated callosal angle measurements screened 4,980 patients in the ADNI and OASIS datasets and identified that 1.8-4.3% of these carefully-screened patients may have treatable NPH.Acknowledgements
We thank the Rudi Schulte Research Institute for grant support for this research.References
1. Bradley WG: Normal Pressure Hydrocephalus: New Concepts on Etiology and Diagnosis. American Journal of Neuroradiology 2000; 21:1586–1590.
2. Ishii K, Kanda T, Harada A, et al.: Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008; 18:2678–2683.
3. Baroncini M, Balédent O, Ardi CE, et al.: Ventriculomegaly in the Elderly: Who Needs a Shunt? A MRI Study on 90 Patients. Acta Neurochir Suppl 2018; 126:221–228.
4. Miskin N, Patel H, Franceschi AM, et al.: Diagnosis of Normal-Pressure Hydrocephalus: Use of Traditional Measures in the Era of Volumetric MR Imaging. Radiology 2017; 285:197–205.
5. Weiner MW, Veitch DP, Aisen PS, et al.: The Alzheimer’s Disease Neuroimaging Initiative 3: Continued innovation for clinical trial improvement. Alzheimer’s & Dementia 2017; 13:561–571.
6. LaMontagne PJ, Benzinger TL, Morris JC, et al.: OASIS-3: Longitudinal Neuroimaging, Clinical, and Cognitive Dataset for Normal Aging and Alzheimer Disease. medRxiv 2019:2019.12.13.19014902.
7. Fischl B: FreeSurfer. Neuroimage 2012; 62:774–781.
8. Virhammar J, Laurell K, Ahlgren A, Cesarini KG, Larsson E-M: Idiopathic normal pressure hydrocephalus: cerebral perfusion measured with pCASL before and repeatedly after CSF removal. J Cereb Blood Flow Metab 2014; 34:1771–1778.
Figures