2367
Cerebrospinal fluid water fraction increases with age in normal aging1Weill Cornell Medicine, New York, NY, United States
Synopsis
Perivascular spaces (PVS) play a key role in the glial-lymphatic waste clearance pathway in the brain. We applied FAST-T2 multi-component T2 relaxometry to 20 healthy volunteers between 30 and 60 years old and found a significant relationship between CSF water fraction and age in the frontal and temporal cortex, while the brain tissue water content measured by T1 relaxation time remains stable over time. Our results suggest that CSF fraction may be a useful quantitative biomarker of PVS dilation in normal aging.
INTRODUCTION
Perivascular spaces (PVS) form a key component of the glial-lymphatic waste clearance pathway for maintaining brain health (1). PVS dilation occurs in normal aging and has been implicated in small vessel disease (2), cerebral amyloid angiopathy, and Alzheimer’s disease (3). Traditionally, PVS enlargement is assessed by counting hyperintense punctate foci in the white matter centrum semiovale and in the basal ganglia on T2-weighted image (4). This method cannot detect the invisible PVS that are much smaller than the imaging voxel. Here we propose a quantitative PVS mapping approach based on multi-component T2 relaxometry which separates the signal of the highly mobile cerebrospinal fluid (CSF) occupying the PVS with very long T2 (~2 sec) from that of restricted water in the brain tissue with much shorter T2 including myelin water (T2<20 msec) and intra/extracellular water (T2~50-80 msec). The Fast Acquisition with Spiral Trajectory and adiabatic T2prep (FAST-T2) sequence can provide whole brain coverage for T2 relaxometry in 4 min (5). The objective of this study was to demonstrate the feasibility of FAST-T2 for mapping of CSF water fraction (CSFF) as a potential quantitative biomarker of PVS dilation and to study the relationship between CSFF and age in healthy volunteers.METHODS
FAST-T2 imaging study. Twenty healthy subjects (mean age 44 years ± 9, range 30-57 years, 11 women, 9 men) underwent brain MRI including a 4 min FAST-T2 sequence with geometric echo spacing for multi-component T2 relaxometry (5) and a 3 min saturation recovery FAST-T1 sequence for T1 mapping (6) on Siemens Skyra 3T scanners. A spatially regularized three-pool non-linear least squares algorithm using the L-BFGS iterative solver was used to extract myelin water fraction (MWF), intra/extracellular water fraction (IEWF), and CSFF maps from the six-echo T2 decay data. The lower and upper T2 bounds for each of the three water pools (in msec) were set to [5 20], [20 200], and [200 2000], respectively. The same solver was used to fit the mono-exponential FAST-T1 data to obtain brain T1 map as a biomarker of tissue water content (7).Image and data analysis. FreeSurfer’s recon-all command was applied to the T1-weighted structural image to obtain brain segmentation and cortical parcellation, which were then aligned to CSFF and T1 maps using a boundary-based registration algorithm (bbregister command) (8). To adjust for multiple comparisons, we performed a multivariate multiple regression with regional CSFF in the frontal, parietal, temporal and occipital cortex and global CSFF in the white matter as the outcomes regressed on age and gender.
RESULTS
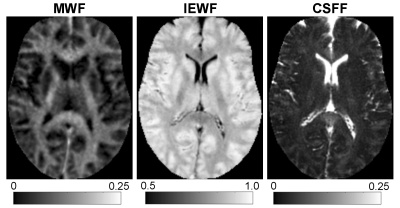
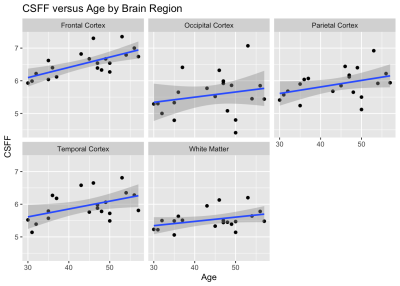
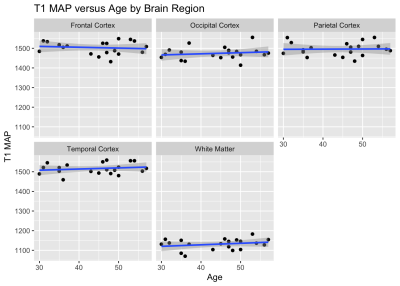
Figure 1 shows an example of the three water fraction maps derived from FAST-T2 which can be interpreted as the relative contribution of water residing within the myelin sheath (myelin water), water residing inside and in-between cells (intra/extracellular water), as well as free water occupying the brain ventricles and PVS. We found statistically significant relationships with age in the frontal (p=0.0011) and temporal (p=0.0093) cortex regions. Holding gender constant, for every year increase in age, CSFF increases on average by 0.03% and 0.02% in the frontal and temporal cortex, respectively (Fig.2). An increasing trend of CSFF with age was observed in the remaining regions (Fig.2); however, the results did not reach statistical significance most likely due to the limited study sample size. The brain tissue water content as measured by T1 map was found to remain stable in this age group (Fig.3).CONCLUSIONS
Our preliminary results suggested that CSF water fraction measured by multi-component T2 relaxometry using the FAST-T2 sequence increases with age in healthy volunteers and may reflect local PVS dilation in agreement with previous findings using the PVS counting method (9). Histopathological validation will be performed to establish CSFF as a quantitative biomarker of PVS dilation in the brain tissue.Acknowledgements
No acknowledgement found.References
1. Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, Tannenbaum A, Ballerini L, Rungta RL, Boido D, Sweeney M, Montagne A, Charpak S, Joutel A, Smith KJ, Black SE; colleagues from the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel Disease. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020;16:137-53.
2. Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, Nedergaard M, Smith KJ, Zlokovic BV, Wardlaw JM. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 2018;114:1462-73.
3. Banerjee G, Kim HJ, Fox Z, Jäger HR, Wilson D, Charidimou A, Na HK, Na DL, Seo SW, Werring DJ. MRI-visible perivascular space location is associated with Alzheimer's disease independently of amyloid burden. Brain 2017;140:1107-16.
4. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010;41(3):450-4.
5. Nguyen TD, Deh K, Monohan E, Pandya S, Spincemaille P, Raj A, Wang Y, Gauthier SA. Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using fast acquisition with spiral trajectory and adiabatic T2prep (FAST-T2) at 3T. Magn Reson Med 2016;76:456-65.
6. Nguyen TD, Spincemaille P, Gauthier SA, Wang Y. Rapid whole brain myelin water content mapping without an external water standard at 1.5T. Magn Reson Imaging 2017;39:82-88.
7. Cercigani M, Dowell NG, Tofts PS. Quantitative MRI of the brain. CRC Press, 2nd Edition, 2018.
8. Fischl B. FreeSurfer. Neuroimage 2012;62:774-81.
9. Feldman RE, Rutland JW, Fields MC, Marcuse LV, Pawha PS, Delman BN, Balchandani P. Quantification of perivascular spaces at 7T: A potential MRI biomarker for epilepsy. Seizure 2018;54:11-18.
Figures