2366
Association of Enlarged Perivascular Spaces and Sleep Disturbances in Military-related Mild Traumatic Brain Injury1National Intrepid Center of Excellence, Bethesda, MD, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Center for Neuroscience and Regenerative Medicine, Bethesda, MD, United States
Synopsis
Sleep disturbances are common following traumatic brain injury (TBI). We sought to quantify changes in perivascular space (PVS) in combat-related mild TBI (mTBI) patients and explore the association between normalized PVS volume and sleep measures. We found mTBI patients with previous potential concussive event (PCE) (TBIPCE) had larger PVS fraction than controls. A subjective sleep measure, The Pittsburgh Sleep Quality Index (PSQI), was positively associated with PVS in TBIPCE patients, which had mean higher PSQI than those of TBI patients who had only mTBI but no PCE. This result suggests enlarged PVS may be modulated by sleep and TBI.
Introduction
Perivascular spaces (PVS) are fluid-filled spaces around cerebral arterioles in the brain parenchyma. They play an important role in the exchange and drainage of interstitial fluid through mechanisms such as the intramural periarterial drainage pathway and the glymphatic system. Such processes may fail after brain injury and accelerate neurodegeneration in the brain. Neuroimaging methods can potentially advance our knowledge of how exchange and drainage of solutes occur through these spaces in the brain. We hypothesize that enlarged PVS is modulated by sleep and traumatic brain injury (TBI), and have implications for the role of the brain clearance system in TBI. The goal of this study is to analyze between-subject variability in the MRI signal reflecting changes in perivascular space volume in mild TBI (mTBI) and their associations with sleep disturbances.Methods
Diagnosis of TBI during enrollment occurred via a multi-layered structured interview process screening for every potential concussive event (PCE) during military deployments and across the entire lifetime, including childhood, using a modification of the Ohio State University TBI Identification (OSU TBI-ID) instrument. 1 Each injury is classified during a consensus meeting as a TBI, potential concussive event (PCE), or no-injury. Depending on the events of TBI and PCE, participants were further grouped into TBIPCE (having both TBI and PCE events), TBIonly (TBI events only), noTBInorPCE (no TBI or PCE events), PCEonly (PCE events only) subgroups. mTBI was diagnosed as previously described. 2 In this retrospective study, we included 172 male service members (88 TBIPCE males, age: 40.9 ± 5.1 years old; 84 TBIonly males, age: 40.7 ± 5.7 years old) and 43 healthy controls (age: 35.5 ± 8.2 years old). Participants underwent MRI scans including structural MRI (T1w, T2w, FLAIR) on a 3T MRI scanner. PVS segmentation was performed using methods similar to those previously described. 3 Briefly, structural MRI data were preprocessed by correcting intensity inhomogeneity and co-registered to ensure correspondence, and adaptive non-local mean filtering was applied. 4 T1w images were segmented into distinct three tissues, gray matter, white matter and cerebrospinal fluid using FSL (https://www.fmrib.ox.ac.uk/fsl). To extract PVS, an Enhanced PVS Contrast image was obtained by dividing the filtered structural scans (T1w/T2w). Vesselness maps were obtained through Frangi filtration 5 and then PVS was automatically segmented and quantified using optimal thresholding of the maps, the 75thpercentil + 1.5 times the InterQuartile Range. The PVS volume fraction for each brain was calculated by dividing the PVS volume by the whole brain volume. General linear models were then applied to regional PVS volume fractions to calculate the main effect of the group while controlling for the covariate, i.e. age. Tukey’s test was applied to correct multiple comparisons. Sleep data (TBI group only), including subjective sleep measure, The Pittsburgh Sleep Quality Index (PSQI), and self-report post-traumatic stress disorder CheckList – Civilian Version (PCL-C) were used for regression and correlation analysis. A cut-off score of PSQI ≥10 was used to indicate a clinically significant sleep disorder. 6Results
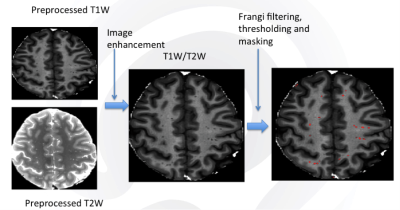
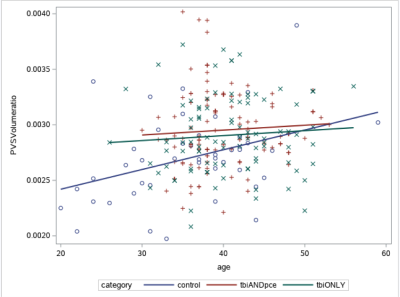
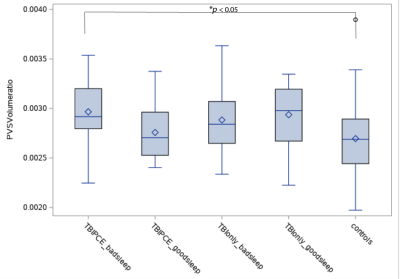
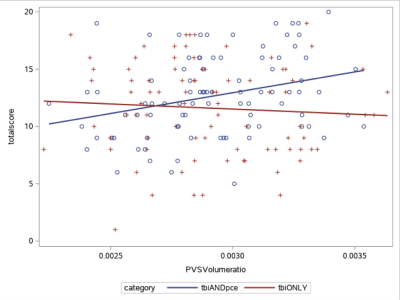
Fig. 1 shows the example images of PVS preprocessing and automated segmentation. Controls were significantly younger than TBI participants (p < 0.05). Controls had lower PCL-C total score (mean=18.5 ± 2.3) than both TBI groups (TBIPCE: 42.8 ± 13.6 TBIonly: 41.2 ±13.3) (p < 0.01). Using a cut-off score of PSQI ≥10, 70 out of 88 (79.5%) mTBI participants in TBIPCE were classified as bad sleepers with an average mean of PSQI of 12.7 ± 3.5; and 55 out of 84 (65.4%) mTBI participants in TBIonly were classified as bad sleepers with an average mean of PSQI of 11.6 ± 4.0. TBIPCE had higher PSQI than that of TBIonly (p = 0.03). PSQI was significantly correlated with PCL-C total score (p < 0.001) in TBI participants. Total brain PVS volume fraction positively correlated with age in controls, but not in TBI subgroups (Fig. 2). PCL-C total score was not a significant confounder to PVS volume fraction. For group comparisons of PVS volume fraction, the TBIPCE bad sleepers subgroup had significantly higher PVS volume fraction than controls (0.296% vs 0.273%, corrected p=0.03) (Fig. 3). In addition, PVS volume fraction positively correlated with PSQI score in TBIPCE subgroup (p=0.0026, r2= 0.13) (Fig. 4), but not in TBIonly subgroup. However, PVS volume fraction was not significantly correlated with numbers of TBI or PCE.Discussion / Conclusions
Our results show that the fraction of whole-brain volume occupied by PVS increases significantly with age in normal individuals but not in TBI patients. It has been shown that aging is associated with dysfunction of the clearance system. 7 It is unclear why PVS volume fraction correlated with subjective sleep PSQI only in TBIPCE but not in the TBIonly group. One explanation is that other neuropsychological disorders, such as stroke, small vessel disease and cognitive impairment 8, can impair the efficacy of brain clearance system and thus result in enlarged PVS. A previous study has shown the association between regional enlarged PVS, e.g. basal ganglion, and PSG sleep measures. 9 In summary, our preliminary results have supported that enlarged PVS may be modulated by sleep and TBI, which suggests a potential application of using routine structural MRI in assessing TBI patients with sleep disturbances.Acknowledgements
Disclaimer
Views expressed in this abstract are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Navy, Army or Defense, or the U.S. Government.
Acknowledgements
We thank the OSU-TBI Group, Theresa Woo, Helena Wu, Cora Davis, Amy Conway, Amanda Gano, Wendy Petit, Chandler Rhodes, Rebecca Sandlain, Tara Staver; NEUROIMAGING Group, Wei Liu, Adam Cliffton, Joseph Hindinger, Chihwa Song at the National Intrepid Center of Excellence, Walter Reed National Military Medical Center for their help on this project. We thank all participants for their participation in this study.
References
1. Walker W. C. et al. "The Chronic Effects of Neurotrauma Consortium (CENC) Multi-Center Observational Study: Description of study and characteristics of early participants," Brain Injury, vol.30:1469-1480, 2016.
2. J Head Trauma Rehabil 1993;8(3):86-87
3. Sepehrband F. et al. “Image processing approaches to enhance perivascular space visibility and quantification using MRI,” Sci. Rep. vol. 9: 12351, 2019
4. J. V Manjón, P. Coupé, L. Martí‐Bonmatí, D. L. Collins, and M. Robles, “Adaptive non‐local means denoising of MR images with spatially varying noise levels,” J. Magn. Reson. Imaging, vol. 31, no. 1: 192–203, 2010.
5. Frangi A. F., Niessen W. J., Vincken K. L., and Viergever M. A., “Multiscale vessel enhancement filtering,” in International Conference on Medical Image Computing and Computer-Assisted Intervention, 1998, pp. 130–137.
6. Matsangas P. & Mysliwiec V., "The utility of the Pittsburgh Sleep Quality Index in US military personnel," Military Psychology, 30:4, 360-369, 2018.
7. Kress B. T. et al., “Impairment of paravascular clearance pathways in the aging brain,” Ann. Neurol., 76: 6, 845–861, 2014.
8. MacLullich A.M., Wardlaw J.M., Ferguson K.J., Starr J.M., Seckl J.R., Deary I.J, "Enlarged perivascular spaces are associated with cognitive function in healthy elderly men," J Neurol Neurosurg Psychiatry. 75(11):1519–1523, 2004.
9. Opel R.A. et al, " Effects of traumatic brain injury on sleep and enlarged perivascular spaces," J Cereb Blood Flow Metab. 2019 Nov; 39(11): 2258–2267.
Figures