2364
Neuropathologic and Cognitive Correlates of Automatically Segmented Enlarged Perivascular Spaces in Community-based Older Adults1Illinois Institute of Technology, Chicago, IL, United States, 2Rush University Medical Center, Chicago, IL, United States
Synopsis
Perivascular spaces form a network that enables clearance of waste products from the brain. Abnormal enlargement of perivascular spaces is common in older adults and has been linked to cognitive impairment and dementia. However, the neuropathologic and cognitive correlates of enlarged perivascular spaces (EPVS) are not well understood yet. In this work, we first developed an algorithm to automatically segment and quantify EPVS in brain MR images, and then investigated the neuropathologic correlates of total and regional EPVS, as well as the contributions of EPVS on cognitive decline in a large community-based cohort of 817 older adults.
INTRODUCTION
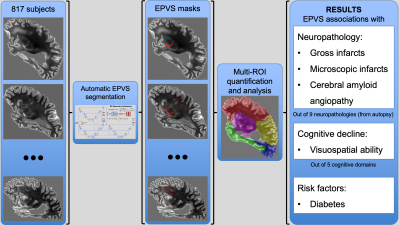
Enlarged perivascular spaces (EPVS) are common in aging and have been linked to increased risk of stroke1, infarcts and diabetes2, lower cognitive function3, and dementia4. However, previous studies have relied almost exclusively on manual semiquantitative assessment of EPVS burden and low numbers of participants, and had limited access to neuropathology and longitudinal cognitive assessments. Therefore, the neuropathologic and cognitive correlates of EPVS are not well understood yet. In this work, we first developed a deep learning model to automatically segment EPVS on MRI. We then combined total and regional EPVS measurements with detailed neuropathology data and longitudinal cognitive assessments on a large number (N=817) of community-based older adults to investigate the neuropathologic correlates of EPVS, as well as the contributions of EPVS to cognition (Fig.1).METHODS
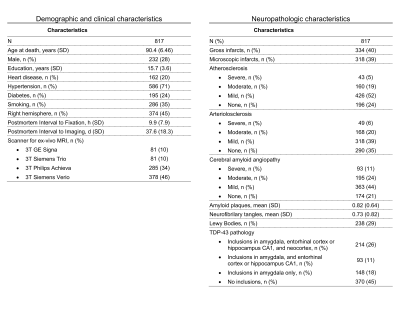
Participants and data:This work included 817 participants of the Rush Memory and Aging Project5, Religious Orders Study6, and Minority Aging Research Study7, three longitudinal cohort studies of aging (Fig.2). All participants underwent annual cognitive assessment. After death, T2-weighted images were collected ex-vivo for one brain hemisphere from each participant using a clinical 3T MRI scanner and a spin-echo sequence with 0.6x0.6x1.5 mm3 voxel-size. Following ex-vivo MRI, all hemispheres underwent detailed neuropathologic examination by a board-certified neuropathologist blinded to clinical and imaging findings (Fig.2). The pathologies assessed included: gross and microscopic infarcts, atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy (CAA), amyloid plaques, neurofibrillary tangles, Lewy bodies, and TDP-43.
Segmentation by deep learning:
Images from 10 participants with a wide range of EPVS burden and manually segmented EPVS were used for training convolutional neural networks (CNNs) to automatically segment EPVS (Fig.3). Models were optimized to maximize performance and prevent overfitting. Model optimization involved network architecture, input features, and multiple regularization strategies. The trained networks were used to segment EPVS in the ex-vivo MRI data of all participants.
Model performance validation:
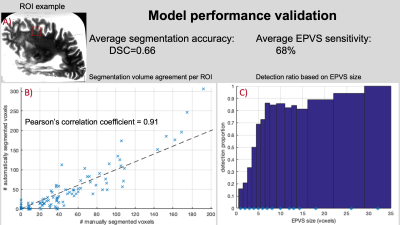
A total of 100 square regions of interest (ROIs) were randomly generated, each on a unique participant outside the training dataset. In each ROI, EPVS were simply identified by an expert neuroradiologist and manually segmented by an experienced observer. Both the neuroradiologist and observer were blinded to model segmentation and to each other. Model segmentation accuracy and sensitivity were evaluated.
EPVS quantification:
The total number of EPVS clusters was counted in each participant. The numbers of EPVS per lobe and in basal ganglia were also counted. All EPVS measurements were log-transformed to reduce skewness.
Statistical analysis:
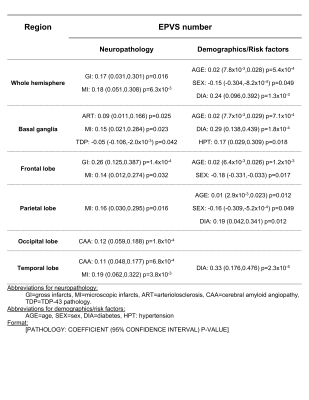
Elastic-net regularized linear regression was used to investigate associations of the number of EPVS (both total and regional) with all neuropathologies (independent variables) controlling for demographics, postmortem interval to fixation and to imaging, and scanner.
Linear mixed-effects models were used to investigate the independent association of EPVS with the rate of cognitive decline above and beyond what was explained by neuropathologies and demographics. This analysis was repeated for global cognition and five cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial abilities.
RESULTS
Validation based on the neuroradiologist showed that the model sensitivity was 68% for all EPVS and >80% for EPVS larger than 5 voxels (Fig.4). Validation of the automated segmentation by an experienced observer showed a Dice similarity coefficient of DSC=0.66. Additionally, the volume in the model-based segmentation was highly correlated (Pearson, ρ=0.91) with that in the manual segmentation per ROI (Fig.4).The total number of EPVS was associated with gross and microscopic infarcts and diabetes (Fig.5). Regional EPVS were associated with infarcts and diabetes in most lobes, and with CAA in the occipital and temporal lobes (Fig.5). Total and frontal lobe EPVS were associated with faster decline in visuospatial ability, (-0.0079, p=0.036) and (-0.0071, p=0.018) respectively, independent of neuropathologies, diabetes and demographics.
DISCUSSION
The present work represents the largest investigation of the neuropathologic and cognitive correlates of EPVS conducted to date, combining full EPVS quantification with detailed neuropathologic examination, and longitudinal cognitive assessment, in a large number of community-based older adults. This study generated robust evidence that EPVS in most of the brain are associated with infarcts, and that EPVS in occipital and temporal lobes are associated with CAA, suggesting that EPVS may share similar neurobiological pathways with these two pathologies. Although the exact underlying mechanisms are currently unknown, the various etiologies that have been proposed for EPVS may also precipitate infarction8,9. Also, amyloid deposition in cortical vessels may impair interstitial fluid drainage, causing retrograde dilation of perivascular spaces10,11. The association of EPVS with diabetes independently of neuropathologies adds important new evidence to recent findings in animal studies implicating diabetes in impairment of the glymphatic system12. Finally, the present work demonstrated independent contributions of EPVS on cognitive decline above and beyond the contributions of neuropathologies and demographics, suggesting that EPVS may capture additional tissue damage not explained by neuropathologies.CONCLUSION
The present investigation provides robust evidence on the neuropathologic correlates of EPVS and the contribution of EPVS on cognitive decline in a large community-based cohort of older adults. Fully quantitative assessment of EPVS was facilitated by deep learning-based segmentation. EPVS were shown to have associations with infarcts, CAA, and diabetes, and independent contributions on cognitive decline above and beyond the effects of known neuropathologies.Acknowledgements
We thank the participants and staff of the Rush Memory and Aging Project, Religious Orders Study, and Minority Aging Research Study.
Sources of Funding:
This study was supported by National Institutes of Health grants P30AG010161,UH2NS100599, UH3NS100599, R01AG015819, R01AG064233, RF-1AG022018, R01AG056405, R01AG042210, R01AG17917, K08NS089830.
References
- Doubal FN, MacLullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM. "Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease". Stroke. 2010;41(3):450-454. doi:10.1161/STROKEAHA.109.564914
- Javierre-Petit C, Schneider JA, Kapasi A, et al. "Neuropathologic and Cognitive Correlates of Enlarged Perivascular Spaces in a Community-Based Cohort of Older Adults". Stroke. 2020;51(9):2825-2833. doi:10.1161/STROKEAHA.120.029388
- MacLullich AMJ. "Enlarged perivascular spaces are associated with cognitive function in healthy elderly men". J Neurol Neurosurg Psychiatry. 2004;75(11):1519-1523. doi:10.1136/jnnp.2003.030858
- Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. "Dilatation of the Virchow-Robin Space Is a Sensitive Indicator of Cerebral Microvascular Disease: Study in Elderly Patients with Dementia". Published online 2005:9.
- Bennett DA, A. Schneider J, S. Buchman A, L. Barnes L, A. Boyle P, S. Wilson R. "Overview and Findings from the Rush Memory and Aging Project". Curr Alzheimer Res. 2012;9(6):646-663. doi:10.2174/156720512801322663
- Bennett DA, A. Schneider J, Arvanitakis Z, S. Wilson R. "Overview and Findings from the Religious Orders Study". Curr Alzheimer Res. 2012;9(6):628-645. doi:10.2174/156720512801322573
- Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. "The Minority Aging Research Study: Ongoing Efforts to Obtain Brain Donation in African Americans without Dementia". Curr Alzheimer Res. 2012;9(6):734-745. doi:10.2174/156720512801322627
- Brown R, Benveniste H, Black SE, et al. "Understanding the role of the perivascular space in cerebral small vessel disease". Cardiovasc Res. 2018;114(11):1462-1473. doi:10.1093/cvr/cvy113
- Wardlaw JM, Smith C, Dichgans M. "Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging". Lancet Neurol. 2013;12(5):483-497. doi:10.1016/S1474-4422(13)70060-7
- van Veluw SJ, Biessels GJ, Bouvy WH, et al. "Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces". J Cereb Blood Flow Metab. 2016;36(3):576-580. doi:10.1177/0271678X15620434
- Charidimou A, Jaunmuktane Z, Baron J-C, et al. "White matter perivascular spaces: An MRI marker in pathology-proven cerebral amyloid angiopathy?" Neurology. 2014;82(1):57-62. doi:10.1212/01.wnl.0000438225.02729.04
- Jiang Q, Zhang L, Ding G, et al. "Impairment of the glymphatic system after diabetes". J Cereb Blood Flow Metab. 2017;37(4):1326-1337. doi:10.1177/0271678X16654702
Figures