2358
Brain Parenchymal Venous System Plays a Substantial Role in Cerebral Waste Clearance1Department of Neurology, Wayne State University School of Medicine, Detroit, MI, United States, 2Department of Radiology, Wayne State University School of Medicine, Detroit, MI, United States, 3Department of Neurology, Henry Ford Health System, Detroit, MI, United States, 4Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States
Synopsis
The current understanding of cerebral waste clearance (CWC) involves CSF participation but lacks the direct participation of the parenchymal vascular system. We used SPIO-enhanced SWI on rats to simultaneously study parenchymal veins, arteries and their corresponding para-vascular spaces, and observed not only CSF participation, but also parenchymal venous participation in CWC following intra-cisterna magna infusion of CSF tracers. These results lead to the speculation of a new CWC mechanism with directional brain parenchyma-to-blood permeability. The substantial role of the parenchymal venous system in CWC raises intriguing questions on the differential contributions of parenchymal venous versus CSF pathways in neurodegenerative disease.
Introduction
Cerebral waste clearance (CWC) has been associated with many physiological and pathophysiological neurological conditions; making it an important area of study lately.1-3 Theoretically, in the brain parenchyma, biochemically-inert substances (i.e. MRI contrast agents) can be removed through two possible pathways: CSF and vascular. Despite the controversy that exists regarding the CSF efflux pathways, there is a solid consensus on the participation of the CSF pathway in CWC. However, the discussion of the brain parenchymal vascular pathway participation seems to pass unacknowledged in most CWC studies, mainly due to the lack of an imaging tool that quantitatively measures tracer influx and efflux in micro-vessels and their surroundings.4, 5We have developed a new MRI method, superparamagnetic iron oxide-enhanced susceptibility weighted imaging (SPIO-SWI)6, 7 which enables the detection of sub-voxel micro-vessels by capitalizing on the SWI blooming effect and the SPIO contrast agent’s high susceptibility. This technique makes it possible to study brain parenchymal vascular participation in CWC. Therefore, the objective of this study was to determine whether CSF MRI tracers in the brain parenchyma are associated more with parenchymal veins than with parenchymal arteries using the SPIO-SWI method.
Methods
Animal: All experimental animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals8 and approved by the local IACUC. A total of 12 healthy adult male/female Wistar and Sprague-Dawley rats were used. Rats were anesthetized with 3.5% isoflurane.Data Acquisition: Intravenous or intra-cisterna magna (ICM) infusion of multiple SPIO tracers (Ferumoxytol [diameter: 17~31nm], FeREX [diameter: 50~150nm], or Fe-dextran [diameter: 100nm]) were performed during MRI experiment. The original iron concentrations were 30 mg/mL in Ferumoxytol, 10mg/mL in FeREX and 2.4 mg/mL in Fe-dextran. MRI measurements were performed with a 7T Bruker small animal system. The dynamic tracer influx and clearance process was monitored using a multi-echo SWI scan as previously described.9 The imaging resolution was 41.6 × 83.2 × 160 μm3.
Data Analysis: MRI data were processed using the SPIN software. Arterial related parameters were measured using the first echo images with better time-of-flight effects and venous related parameters were measured using the second echo images with better susceptibility effects. MRI signal intensity (SI) was measured as a sum over all voxels in the 3D-ROIs of arteries and veins, respectively. Post-contrast SI changes relative to the baseline scan were presented as mean (±SE) and were plotted as appropriate.
Results
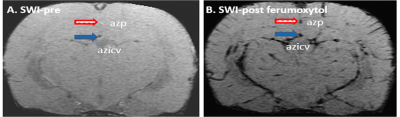
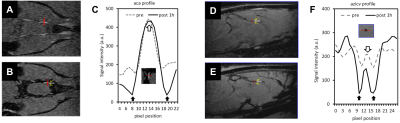
As shown in Fig-1, the SPIO-SWI method distinguished parenchymal arteries and veins in rats. Following an ICM infusion of Ferumoxytol, a time series of SPIO-SWI scans showed a dynamic tracer pattern (Fig-2) consistent with the previous study.2 These findings are consistent with established CSF pathways,1, 2, 10, 11 and suggest that our procedures do not disrupt the system.In order to determine whether there is a net influx of MRI tracers from the brain parenchyma into the vascular system, we first qualitatively evaluated whether the amount of MRI tracers in the brain parenchymal veins was more than that in the brain parenchymal arteries (Fig-3). The result clearly distinguished blood vessels from para-vascular spaces; and demonstrates that the MRI tracers flowed along the para-arterial space but did not enter the arterial blood (Fig-3C); and MRI tracers flowed along the para-venous space and entered in the venous blood (Fig-3F).
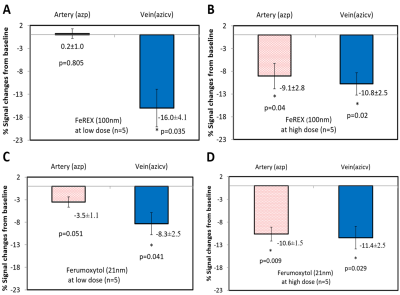
We quantitatively measured CSF tracer entry into the azygos pericallosal artery (azp) and the azygos internal cerebral vein (azicv). A low dose of FeREX (Fig-4A) resulted in significantly different SI changes in venous and arterial blood: 16.0(±4.1)% versus 0.2(±1.0)%; which were comparable for the high dose of FeREX experiment (Fig-4B). Similar results were obtained using Ferumoxytol, a smaller MRI tracer (Fig-4C,D).
Discussion and Conclusions
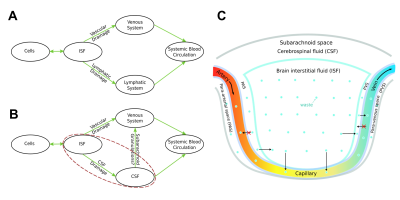
In this study, we demonstrated that there was a significant brain parenchyma-to-blood efflux of MRI tracers into the parenchymal venous system following ICM infusion of tracer, suggesting that there may be directional differences in BBB permeability, as these MRI tracers are established as blood-to-brain parenchyma impermeable via the BBB, which supports parenchymal vascular system involvement in CWC. The findings that MRI tracers cross the BBB via the parenchymal venous system but not via the parenchymal arterial system is logically consistent with the differences in the anatomical structures and functions between the venous and arterial systems. These findings led us to the speculation of an unknown CWC mechanism: non-specific wastes can enter into the parenchymal venous blood from the brain parenchyma efficiently, and it is a one-way transfer (Fig-5).The direct participation of the parenchymal venous system questions our traditional understanding of the role of arachnoid granulations in CWC. CSF involved with CWC drains into the systemic blood circulation via two routes; either directly through dural venous sinuses via arachnoid granulations, or indirectly through lymphatic pathways.12-14 If waste in the brain parenchyma can directly enter the parenchymal venous system, and since blood circulation is much faster than CSF circulation, we can reasonably surmise that the arachnoid granulations are at most, if at all, only a secondary pathway for CWC; further challenging the traditional understanding of the role of arachnoid granulations in CWC (Fig-5B).
We conclude that there is parenchymal venous participation in CWC, complementary to established CSF pathways.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) (Grant Numbers: RF1-AG057494, R01-NS108463 and R01-NS108491).References
1. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012;4:147ra111.
2. Jiang Q, Zhang L, Ding G, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab 2017;37:1326-1337.
3. Louveau A, Da Mesquita S, Kipnis J. Lymphatics in Neurological Disorders: A Neuro-Lympho-Vascular Component of Multiple Sclerosis and Alzheimer's Disease? Neuron 2016;91:957-973.
4. Sherwood L. Human Physiology: From Cells to Systems, 7th ed2010.
5. Haughton VM, Korosec FR, Medow JE, Dolar MT, Iskandar BJ. Peak systolic and diastolic CSF velocity in the foramen magnum in adult patients with Chiari I malformations and in normal control participants. AJNR Am J Neuroradiol 2003;24:169-176.
6. Wang H, Jiang Q, Shen Y, et al. The capability of detecting small vessels beyond the conventional MRI sensitivity using iron-based contrast agent enhanced susceptibility weighted imaging. NMR Biomed 2020;33:e4256.
7. Shen Y, Hu J, Eteer K, et al. Detecting sub-voxel microvasculature with USPIO-enhanced susceptibility-weighted MRI at 7 T. Magn Reson Imaging 2020;67:90-100.
8. NIH. Guide for the Care and Use of Laboratory Animals, 8th Edition. Washington (DC): National Academies Press (US), 2011.
9. Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging 2015;33:1-25.
10. Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 2004;1:2.
11. Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide. Neurochem Res 2015;40:2583-2599.
12. Weed LH. Studies on Cerebro-Spinal Fluid. No. III : The pathways of escape from the Subarachnoid Spaces with particular reference to the Arachnoid Villi. J Med Res 1914;31:51-91.
13. Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 2017;8:1434.
14. Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res 2010;7:9.
Figures