2350
Evaluation of Microvascular Invasion of Hepatocellular Carcinoma by Using R2* mapping of Chemical shift encoded MRI1Radiology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China, 2University of Wisconsin, Madison, WI, United States
Synopsis
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related mortality. Microvascular invasion (MVI) of HCC is a major prognostic factor that influences treatment strategy and long-term survival, but it is difficult to identify MVI until the tumor is surgically removed and analyzed histologically. Both DCE-MRI and Chemical shift encoded (CSE) MRI can be used to evaluate of HCC in clinical practice. Using CSE-MRI, we found a high percentage of elevated peritumoral R2* on quantitative R2* maps with MVI-positive patients compared to MVI-negative patients. CSE-MRI has the potential to assess MVI of HCC preoperatively.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third most common cause of cancer-related mortality 1. Microvascular invasion (MVI) of HCC is a major prognostic factor that influences the suitability of surgery 2. Preoperative assessment of MVI using a non-invasive method would be helpful to adapt treatment strategy and could impact long-term survival. Chemical shift encoded (CSE) MRI is a potential method to evaluate of intra-tumoral fat in HCC 3, 4, but also assesses tissue R2*. The purpose of this study was to investigate whether high signal distribution around the tumor on R2* of CSE-MRI can preoperatively predict MVI of HCC.Methods
This retrospective study was approved by the Institutional Review Board of our institution. From January 2018 to April 2019, 88 patients proven HCC by surgery were included in our study, who underwent preoperative 3.0 T MRI examination.A 3D quantitative chemical shift encoded MRI (CSE-MRI) method (IDEAL-IQ, GE, Discovery MR750, Waukesha, WI) acquired within a single 24s breath-hold in all patients. This method automatically generates proton density fat fraction (PDFF)and R2* maps. Acquisition parameters for IDEAL IQ included: FA 8°; TR/TE = 7.9/0.9ms, FOV = 400mm*320mm; slice thickness = 8 mm, data matrix = 128*128; NEX = 0.75. Increasing flip angles were chosen to increase the relative signal from fat and therefore increase the SNR of the fat signal. Although this leads to T1-related bias in the fat-fraction measurement, we hypothesized that the improvement in SNR performance may improve the quantification of R2*. Elevated peritumoral R2* of CSE-MRI were assessed independently according to by two abdominal radiologists with 6 years and 15 years of experience in liver MRI, respectively, unaware of clinical, laboratory, pathologic, and follow-up information. Disagreements were resolved by consensus. HCCs were diagnosed and MVI was assessed by a liver pathologist with 14 years of experience on hematoxylin-and-eosin-stained slides. Kappa test was performed to assess MRI features for predicting MVI. Statistical significance was defined as P<0.05.Results
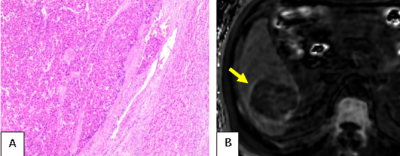
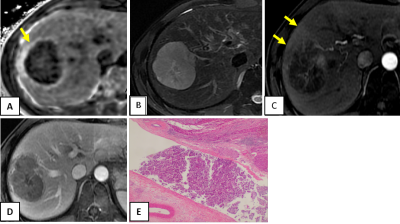
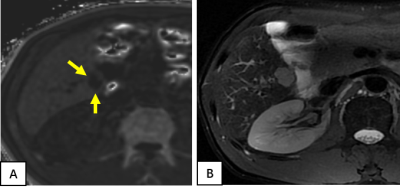
Among the 88 analyzed HCC tumors, the average size was 41 mm (range =15-89mm). Pathological specimens were categorized as without MVI in 40 tumors, and with microvascular invasion in 48 tumors. 45 (94%) tumors out of 48 with MVI show elevated peritumoral R2* (Figures 1 and 2), while 13 (32%) tumors out of 40 without MVI of HCCs showed elevated peritumoral R2*(Figures 3). The percentage of elevated peritumoral R2* of CSE-MRI for MVI-positive HCCs was significantly higher than for MVI-negative HCCs(kappa=0.626,P<0.05).Discussion
HCC is the only tumor that can be diagnosed noninvasively by imaging-based criteria without confirmatory biopsy. Our study suggests that high signal distribution around the tumor on R2* can predict MVI of HCC. We hypothesize that arterial-portal shunting caused by MVI may explain abnormal peritumoral iron deposition. If validated by future independent studies, such information may inform optimal surgical decision, especially for patients with contraindications to contrast medium examination. Limitations of our study include that it was single-center and retrospective with a small number of cases, and focused only on 3.0 T MRI.Conclusion
Our preliminary results suggest that elevated peritumoral R2* measured using CSE-MRI can potentially predict MVI of HCC.Acknowledgements
The authors state that this study has received funding by National Natural Science Foundation of China grant 91959118 (JW), Science and Technology Program of Guangzhou, China 201704020016 (JW) and Clinical Research Foundation of the 3rd Affiliated Hospital of Sun Yat-Sen University YHJH201901 (JW).References
1. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362(9399):1907–1917.
2. Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017;67(3):526-534.
3. Siripongsakun S, Lee JK, Raman SS, Tong MJ, Sayre J, Lu DS. MRI detection of intratumoral fat in hepatocellular carcinoma: potential biomarker for a more favorable prognosis. AJR Am J Roentgenol. 2012;199(5):1018-25.
4. Min JH, Kim YK, Lim S, Jeong WK, Choi D, Lee WJ. Prediction of microvascular invasion of hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging: Impact of intra-tumoral fat detected on chemical-shift images. Eur J Radiol. 2015;84(6):1036-43.
Figures