2348
The valuation of MRI based on IDEAL-IQ in differential diagnosis between HCC with Negative Alpha Fetal Protein and FNH1Department of Radiology, The First Affiliated Hospital of USTC, Southern District of Anhui Provincial Hospital, Hefei, China, 2The First Affiliated Hospital of USTC, Southern District of Anhui Provincial Hospital, hefei, China
Synopsis
To investigate the MRI technique based on the sequence of IDEAL-IQ (iterative decomposition of water and fat with echo asymmetry and least squares quantification sequence) in differential diagnoses between Hepatocellular Carcinoma with Negative Alpha Fetal Protein and Hepatic Focal Nodular Hyperplasia (FNH).The fat fraction and R2* value measured by IDEAL-IQ sequence are useful to distinguish HCC with Negative Alpha Fetal Protein from FNH.
1
Objective To investigate the MRI technique based on the sequence of IDEAL-IQ (iterative decomposition of water and fat with echo asymmetry and least squares quantification sequence) in differential diagnoses between Hepatocellular Carcinoma with Negative Alpha Fetal Protein and Hepatic Focal Nodular Hyperplasia (FNH).Methods To analyse retrospectively the clinical and imaging materials of 15 patients with AFP negative HCC and 7 patients with FNH proven by operation and pathology during July 2019 to October 2020. All patients underwent IDEAL-IQ sequence scanning before operation. The fat content and iron content of liver tissue and lesion in the two groups were measured by fat ratio image and R2 * relaxation rate image and analyse the differences of fat content and iron content between the two groups. The diagnostic efficacy of fat content and iron content in the two groups were compared by curve analysis.
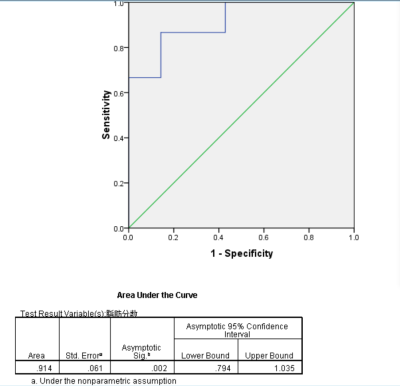
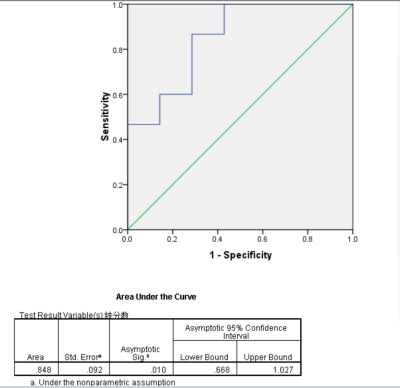
Results The mean fat fraction of HCC was 8.146± 5.993%, and that of FNH was 2.359±1.267%, with significant difference between the two groups. The mean value of R2* of liver and lesion in HCC group was 21.513±5.491Hz、 60.223±11.942Hz, the mean value of R2* of liver/lesion was 3.159±1.247.The mean value of R2* of liver and lesion in FNH group was 41.178±3.324Hz、21.533±5.491Hz,and the mean value of R2* in liver / lesion was 2.014±0.441. There was no significant difference in R2* value between HCC and FNH, but there was significant difference in R2* value of liver tissue and R2 * value of liver / lesion between the two groups. The areas under the ROC curve of fat fraction was 0.914, and take 2.67% as cut-off value the sensitivity and specificity for diagnosis of HCC were 86.7% and 85.7%; the areas under the curve of R2* value of liver/lesion was 0.848, and take 2.15 as cut-off value the sensitivity and specificity for diagnosis of HCC were 86.7% and 71.4%.
Discussion The development of hepatocellular carcinoma (HCC) in cirrhotic liver is usually described as a process of hepatocarcinogenesis, from dysplastic nodule (DN), to DN with microscopic foci of HCC (DN-HCC), and finally to overt HCC. Iron overload is another common pathophysiological change in cirrhotic liver. Excessive iron accumulation may be a causative factor of chronic liver disease progression and hepatocarcinogenesis. HCCs arising in siderotic liver tend to be iron-deficient at pathological analysis and MR examination. Hepatic Focal Nodular Hyperplasia (FNH) is mainly composed of normal cells such as hepatocytes, which does not belong to neoplastic lesions. The decrease in iron content is averaged. About fat, the presence of fat in FNH is extremely rare and may or may not be associated with diffuse hepatic steatosis. However, fatty change can be seen in up to 35% of small HCCs and is associated with a decrease in the number of intratumoral arteries without any difference in intratumoral portal tracts. Conclusion The fat fraction and R2* value measured by IDEAL-IQ sequence are useful to distinguish HCC with Negative Alpha Fetal Protein from FNH,and the fat fraction has the best diagnostic efficacy for differentiations.
Acknowledgements
For the collaboration, authors are grateful to QIU JUN ,WANG PENG,DENG KEXUE and WANG JIZHOU.
References
1 Kobayashi M, Ikeda K, Hosaka T, et al. Dysplastic nodules frequently develop into hepatocellular carcinoma in patients with chronic viral hepatitis and cirrhosis. Cancer,2006, 106:636–647.
2. Di Tommaso L, Sangiovanni A, Borzio M, et al. Advanced precancerous lesions in the liver. Best Pract Res Clin Gastroenterol,2013,27:269–84.
3. Gong L, Wei LX, Ren P, et al. Dysplastic nodules with glypican-3 positive immunostaining: a risk for early hepatocellular carcinoma. PLoS One,2014,9:e87120.
4. Sciarra A, Di Tommaso L, Nakano M, et al. Morphophenotypic changes in human multistep hepatocarcinogenesis with translational implications. J Hepatol. 2015,pii:S0168-8278(15) 00611-X.
5. Ludwig J, Hashimoto E, Porayko MK, et al. Hemosiderosis in cirrhosis: a study of 447 native livers. Gastroenterology,1997, 112:882–888.
6. Zhang J, Krinsky GA. Iron-containing nodules of cirrhosis. NMR Biomed,2004, 17:459–464.
7. Terada T, Nakanuma Y. Iron-negative foci in siderotic macroregenerative nodules in human cirrhotic liver. a marker of incipient neoplastic lesions. Arch Pathol Lab Med,1989,113:916–920.
8. Li RK, Suzanne L. Palmer3, Zeng MS, et al. Detection of Endogenous Iron Reduction during Hepatocarcinogenesis at Susceptibility-Weighted MR Imaging: Value for Characterization of Hepatocellular Carcinoma and Dysplastic Nodule in Cirrhotic Liver. PLoS One,2015, 10(11): e0142882.
9. Yoon JH, Lee JM, Yu MH, et al. Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: Comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging,2014,39(2):276-285.
10. Stanley G, Jeffrey RB Jr, Feliz B. CT findings and histopathology of intratumoral steatosis in focal nodular hyperplasia: case report and review of the literature. J Comput Assist Tomogr 2002; 26:815-817. 11.Kutami R, Nakashima Y, Nakashima O, et al. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol 2000; 33:282–289.