2340
Radiomics analysis on SWI in hepatocellular carcinoma: exploring the correlation between histopathology and radiomics features1Sun Yat-sen University Cancer Center, Guangzhou, China, 2Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Susceptibility weighted imaging (SWI) has shown tremendous clinical potential in visualizing the micro-haemorrhage, calcification, neovascularization and so forth, which serve as important micro-structural manifestations during the carcinogenesis. Radiomics is of great significance for providing valuable quantitative image markers via high throughput feature extraction. However, hardly have the radiomics been applied for extracting the quantitative markers from SWI images. This research, hence, aims to extract the diagnostic markers from the SWI images for evaluating several important prognostic markers of hepatocellular carcinoma including histopathologic grade, microvascular invasion (MVI) as well as the expression status of cytokeratin 7, cytokeratin 19 and Glypican-3.
Introduction
Comprehensive insights from histopathologic features are conducive to guide the following diagnosis, therapy and prognosis prediction of hepatocellular carcinoma (HCC). For example, HCCs with positive cytokeratin 19 (CK-19) always indicates a high rate of recurrence, poor prognosis and a high probability of lymph node metastasis.1 By means of sensing the susceptibility differences caused by “intrinsic contrast agents” containing deposited iron, hemoglobin, hemosiderin, susceptibility weighted imaging (SWI) has evolved as an emerging MRI technique for characterizing hepatocellular carcinoma with unique image contrast due to the additional phase information.2 Recently, able to extract thousands of quantitative image features, the state-of-art radiomics technology helps pave the unprecedented way for reflecting the underlying pathology via capturing the pathology related image manifestations invisible to the naked eyes.3 To the best of our knowledge, no previous researches have extracted radiomics features from SWI for biomedical applications. Thus, this research aims to explore the correlations between the SWI-derived radiomics features and multiple histopathologic features of HCC including MVI, cytokeratin 7 (CK-7), cytokeratin 19 (CK-19) and Glypican-3 (GPC-3) and histopathologic grade.Methods
A total of 53 patients were ultimately enrolled into this retrospective study with MR examinations undertaken at a 3T scanner (uMR 780, United Imaging Healthcare, Shanghai, China). The detailed parameters of SWI sequence are as followings: TR/TE/FA:142.3 ms/10.0 ms/30°; Matrix/FOV: 368×216/280×380 mm2; 107 radiomics features were extracted from SWI images of each patient. Then the Spearman correlation test was performed to evaluate the correlation between the SWI-derived radiomics features and histopathologic indexes including histopathologic grade, microvascular invasion (MVI) as well as the expression status of cytokeratin 7 (CK-7), cytokeratin 19 (CK-19) and Glypican-3 (GPC-3). With SWI-derived radiomics features utilized as independent variables, four logistic regression based diagnostic models were established for diagnosing patients with positive CK-7, CK-19, GPC-3 and high histopathologic grade, respectively. Then, receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic performance.Results
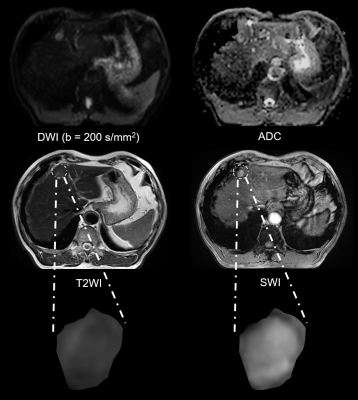
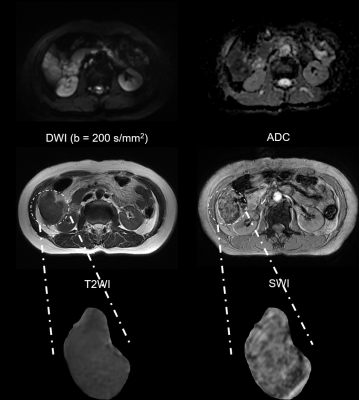
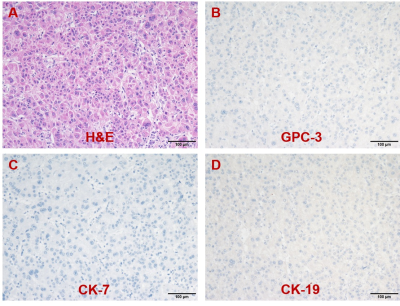
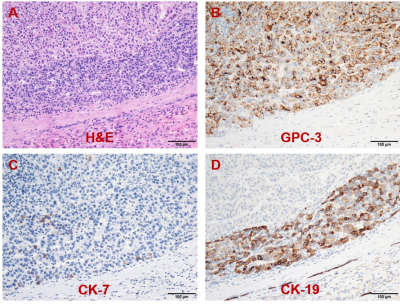
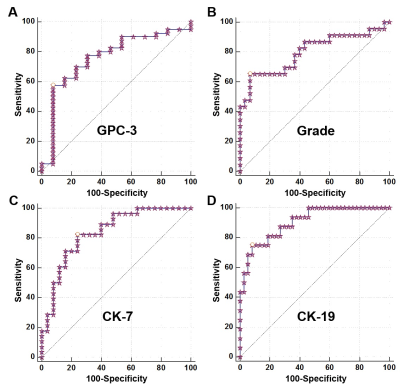
Representative MR images and immunohistochemical staining specimens of two patients with different histopathological features were shown in Figure 1-4. With regard to representative images of a patient with low grade HCC as well as negative status of MVI, GPC-3, CK-7 and CK-19 in Figure 1, SWI images of another patient with high grade HCC as well as positive status of MVI, GPC-3, CK-7 and CK-19 in Figure 2 displayed that there were some irregular hypo-signal intensities which were caused by the extra dephasing effect because of intra-tumoral hemorrhage, hypervascularity and so on. 11, 32, 18 and 1 SWI-derived radiomics features were significantly correlated with histopathologic grade, the expression of CK-7, the expression of CK-19 and the expression of GPC-3 (p < 0.05), respectively. None of the SWI-derived radiomics features was correlated with MVI status. Four binary logistic regression based diagnostic models were established via introducing the SWI-derived radiomics features significantly correlated with specific histopathological indexes. The AUCs were 0.905, 0.837, 0.800 and 0.760 for diagnosing patients with positive CK-19, positive CK-7, high histopathologic grade and positive GPC-3. (Figure 5).Discussion
The results suggested that radiomics features derived from SWI were significantly correlated with the histopathologic grade, the expression status of CK-7, CK-19 and GPC-3, which meant that it was feasible to utilize the SWI-derived radiomics features for evaluating the histopathological features of HCC. The potential causes were as the followings: 1) SWI applies the phase information to enhance the contrast of susceptibility change and is very sensitive for the “intrinsic contrast agents” such as deoxygenated hemoglobin, deposited iron and more. As a result, SWI is able to visualize some pathological changes including nodular dysplasia, hyper-vascularity, hemorrhage, even some specific morphological patterns such as the mosaic pattern of HCC.4 2) The state-of-art radiomics technology provided an unprecedented opportunity for extracting the underlying quantitative insights of images.5,6 Thus, radiomics analysis is powerful for timely capturing and reflecting the potential pathological changes. Based on the aforementioned points, significant correlations between histopathologic features of HCC and SWI-derived radiomics features were obtained with the assistance of unique image contrast of SWI and hundreds of quantitative radiomics parameters. However, none of SWI-derived radiomics features was significantly correlated with the MVI status. Potential reasons were as the follows: compared to “invaded micro-vessel” of patients with MVI which are always located in the adjacent liver tissue and portal vein, SWI is more sensitive for sensing the pathologic changes related to “macro-vessels” such as hypervascularity and hemorrhage.4 As a result, SWI-derived features didn’t show significant correlation with MVI in this research. However, more pathologically relevant evidence needs to be mined in subsequent studies. Our results indicated HCCs with high histopathologic grade, positive CK-19, positive CK-7 and positive GPC-3 can be diagnosed with satisfying accuracy. Above results further proved that: 1) some intrinsic “contrast agents” of SWI can be utilized for indirectly characterizing other histopathologic changes. 2) Integrating multiple radiomics features was meaningful for establishing powerful diagnostic models.Conclusion
In conclusion, this research suggested that extracting the radiomics features from SWI images is feasible to evaluate multiple histopathologic indexes of HCC.Acknowledgements
NoneReferences
1. Choi S-Y, Kim SH, Park CK, et al. Imaging Features of Gadoxetic Acid–enhanced and Diffusion-weighted MR Imaging for Identifying Cytokeratin 19-positive Hepatocellular Carcinoma: A Retrospective Observational Study. Radiology 2018;286(3):897-908.
2. Haacke EM, Xu Y, Cheng YCN, Reichenbach JR. Susceptibility weighted imaging (SWI). Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2004;52(3):612-618.
3. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016;278(2):563-577.
4. Chang S-X, Li G-W, Chen Y, et al. Characterizing venous vasculatures of hepatocellular carcinoma using a multi-breath-hold two-dimensional susceptibility weighted imaging. PloS one 2013;8(6):e65895.
5. Lambin P, Leijenaar RT, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nature reviews Clinical oncology 2017;14(12):749-762.
6. Van Griethuysen JJ, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer research 2017;77(21):e104-e107.
Figures