2332
Deep Learning Denoising to Accelerate Diffusion-Weighted Imaging of Rectal Cancer1Medical physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Deep learning denoising using a convolutional neural network (DnCNN) is proposed to accelerate the acquisition of diffusion-weighted imaging (DWI) data in patients with rectal tumors. 4-fold acceleration was achieved by reducing the number of averages from 16 to 4 and applying the DnCNN to denoise the data. The DnCNN was trained using pairs of noisy (1 average) and reference (16 averages) images from 92 patients. The trained network was then tested with the data from 6 patients and the results were evaluated qualitatively by radiologists. DnCNN represents a powerful approach to improve efficiency of DWI in patients with rectal cancer.
Abstract
Introduction:MRI is the imaging modality of choice for diagnosis and staging of rectal cancer. T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI) are the two most frequent techniques for the evaluation of rectal tumors. In particular, DWI provides tissue cellularity information that can be used to characterize microstructures of rectal tumors and thus to enable early diagnosis and to evaluate the efficacy of neoadjuvant therapy1-6. However, due to the intrinsic low SNR nature of high b-value DWI, several acquisitions need to be performed and averaged to achieve sufficient SNR, at the expense of elongating the examination. For example, it is common to use 16 acquisitions for high b-value DWI. In addition to long scan times, inconsistencies between individual acquisitions can result in artifacts. Therefore, there is an urgent need to speed up the acquisition of rectal DWI data and reduce the number of individual acquisitions to be averaged 7-10.This work presents the application of deep learning denoising11 to reconstruct rectal DWI using fewer acquisitions, where a convolutional neural network (CNN) is learned using pairs of noisy (reduced number of averages) and reference (standard number of averages) images. In addition to the noisy high b-value image, the higher SNR low b-value image was used as a guide to improve the denoising process.
Methods:
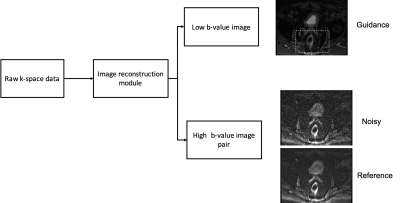
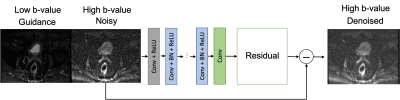
DWI data: Raw k-space data for conventional single-shot EPI DWI rectal acquisition (standard-of-care at our institution) were retrospectively collected from 13 3T MRI scanners (GE Healthcare, Waukesha, WI) with IRB approval. Raw data for b=0 were acquired with NEX=2 (number of averages) and raw data with b=800 s/mm2 were acquired with NEX=16. Note that raw data acquisition enables to reconstruct the data with a user-defined number of averages. Low b-value images were reconstructed with NEX=2 and high b-value images were reconstructed with NEX=1 and 4 (noisy) and NEX=16 (reference for training) (Figure 1). Deep learning denoising: The denoising CNN (DnCNN), which exploits residual learning to separate noise from noisy images, modified to include the low b-value image as an additional input11, was used. The relatively high SNR of the low b-value DWI acquisition was used as a guide for the denoising process. The network was trained with pairs of noisy high b-value DWI (NEX=1) and reference high b-value DWI (NEX=16) from 98 patients (3640 pairs of images). The data from 83 patients, equivalent to 3079 image pairs (84%), were used to train the network, 9 patients equivalent to 335 image pairs (~9%) were used for evaluation of the network and 6 patients or 226 image pairs (~6%) were used for testing. The single output of the network generated the residual image which was subtracted from the noisy image to obtain the denoised image. The loss function was the mean squared error between the denoised image and the reference image. The trained guided DnCNN network with 60x60 patch size and depth of 23 was applied to the test set (Figure 2).
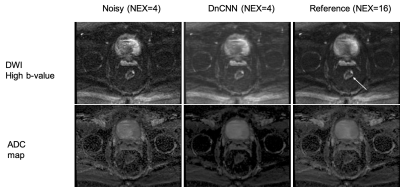
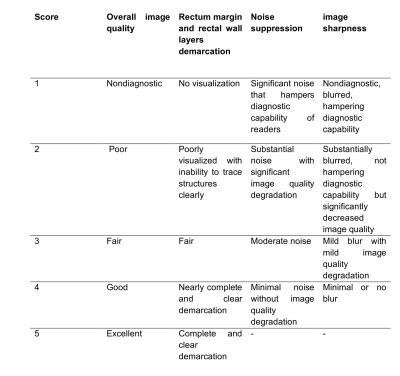
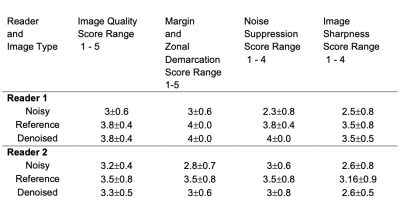
Evaluation: The effect of denoising DWI using guided DnCNN was qualitatively evaluated by two diagnostic body radiologists. The scoring system, based on previous studies, is shown in Table 1. The readers independently scored noisy, reference, and denoised images in a randomized order. Apparent Diffusion Coefficient (ADC) maps were also calculated and presented in addition to DWI (Figure 3).
Results:
The network was trained for 15 epochs, which resulted in about 38 hours of total training time (2.5 hours for each epoch). Testing time, this is the application of the trained network to a testing case, was only 70s. Figure 3 shows the results in one of the testing cases. Both the denoised high b-value image and resulting denoised ADC map compare favorably to the original noisy results and approximate the results obtained with the reference image. The high-value image shows elevated contrast in the tumor and the ADC is lower in the tumor, as expected due to restricted diffusivity. There is a slight blurring in the denoised images, as a result of the denoising process, which uses spatial filters. Table 2 shows the reader scoring results. Denoised images were superior to noisy images in terms of overall image quality, tumor delineation, and noise suppression, and they were similar in terms of image sharpness. The latter is expected since noisy images usually appear sharper than denoised images, as other studies have shown.
Conclusion:
Deep learning denoising enables 4-fold acceleration of rectal DWI, which significantly improves the efficiency of DWI for diagnosis and assessment of treatment response for rectal tumors. While the network training requires a substantial number of data sets and is time-consuming, the application of a trained model is very fast (close to one minute). Future work will focus to apply the deep learning denoising framework for multi-shot EPI with the goal of increasing spatial resolution and reducing geometric distortions.
Acknowledgements
No acknowledgement found.References
1. Smith JJ, Strombom P, Chow OS, et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA oncology. 2019;5(4):e185896.
2. Horvat N, Veeraraghavan H, Pelossof RA, et al. Radiogenomics of rectal adenocarcinoma in the era of precision medicine: A pilot study of associations between qualitative and quantitative MRI imaging features and genetic mutations. European journal of radiology. 2019;113:174-181.
3. Patel UB, Brown G, Rutten H, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19(9):2842- 2852.
4. Sclafani F, Brown G, Cunningham D, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer. 2017;117(10):1478-1485.
5. Siddiqui MR, Gormly KL, Bhoday J, et al. Interobserver agreement of radiologists assessing the response of rectal cancers to preoperative chemoradiation using the MRI tumor regression grading (mrTRG). Clin Radiol. 2016;71(9):854-862.
6.Maas M, Lambregts DM, Nelemans PJ, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol. 2015;22(12):3873-3880.
7. Hotker AM, Tarlinton L, Mazaheri Y, et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A compariso n of morphological, volumetric and functional MRI parameters. European radiology. 2016;26(12):4303-4312.
8. LambregtsDM, RaoSX, SassenS, etal. MRI and Diffusion-weightedMRIVolumetryforIdentification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Annals of surgery. 2015;262(6):1034-1039.
9. Van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269(1):101-112.
10. Van den Broek JJ, van der Wolf FS, Lahaye MJ, et al. Accuracy of MRI in Restaging Locally Advanced Rectal Cancer After Preoperative Chemoradiation. Diseases of the colon and rectum. 2017;60(3):274- 283.
11. Kaye, E. A. et al. Accelerating Prostate Diffusion-weighted MRI Using a Guided Denoising Convolutional Neural Network: Retrospective Feasibility Study. Radiology: Artificial Intelligence 2, e200007 (2020).
Figures