2327
Pseudo continuous arterial spin labeling perfusion MRI for predicting tumor response to neoadjuvant chemotherapy in advanced rectal cancer1Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan, 2Kagoshima University Hospital, Kagoshima, Japan, 3Philips Japan, Minatoku, Japan
Synopsis
This study focused on the feasibility of pseudo continuous arterial spin labeling (pCASL) perfusion MRI as a non-invasive tool for predicting the response of locally advanced rectal cancer treated with neoadjuvant chemotherapy in comparison with dynamic contrast-enhanced MRI and diffusion-weighted imaging. Our results showed blood flow derived from pCASL was significantly higher in responders than in non-responders. These results suggested the promise of pCASL as a non-invasive alternative to dynamic contrast-enhanced MRI for predicting the treatment response to NAC in locally advanced rectal cancer.
Introduction
Neoadjuvant chemotherapy (NAC) followed by total mesorectal excision is the standard treatment for locally advanced rectal cancer. Pharmacokinetic parameters of dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted imaging (DWI) are reportedly useful for predicting tumor response to NAC for locally advanced rectal cancer. Pseudo continuous arterial spin labeling (pCASL) perfusion MRI is a non-invasive, nonradioactive, and non-contrast-enhanced method capable of quantitatively measuring microvascular perfusion characteristics of tissues. However, the usefulness of pCASL for assessing the therapeutic response to NAC in locally advanced rectal cancer has not been elucidated. Therefore, the objective of this study was to evaluate the usefulness of pCASL for predicting the therapeutic response to NAC in locally advanced rectal cancer in comparison with DCE-MRI and DWI.Methods
Our study population consisted of 36 patients (26 men, 10 women; mean age, 63 years; range, 26–75 years) with histologically confirmed locally advanced rectal cancer who underwent 3D-pCASL, DCE-MRI, and DWI before chemotherapy. The pCASL MRI was acquired by 3D volume isotropic turbo spin-echo acquisition using a 3T MRI. The labeling plane was 80 mm above the center of the image FOV. Labeling was applied for 3 sec followed by a 1.6-sec post labeling delay before image acquisition. The total scan time of pCASL examination was 4 min 7 sec. Other pCASL parameters were as follows: TR/TE = 6500/36 ms, slice thickness = 5.0 mm, FOV = 230×230 mm, matrix = 192×192. For the DCE-MRI study, a bolus of gadolinium-DTPA (0.1mmoL/kg) was injected into a vein at an injection rate of 3.0 mL/sec using an automated injector and was followed by a 25-mL saline flush. The temporal resolution of 3D-fast field-echo (FFE) sequence was approximately 3.4 sec, and dynamic data acquisition was started after the contrast medium injection and repeated 95 times. For T1 maps, precontrast 3D-FFE with dual flip angles (5o and 15o) was performed. DWI was acquired by fat-suppressed single-shot echo-planar imaging with diffusion gradients (b = 0 and 1000 sec/mm2). We calculated blood flow (BF) derived from pCASL, DCE MRI parameters, including Ktrans, Kep, Ve, and Vp, and apparent diffusion coefficient (ADC) value from DWI within tumors. Patients were classified as responders or non-responders according to Response Evaluation Criteria in Solid Tumors.Results
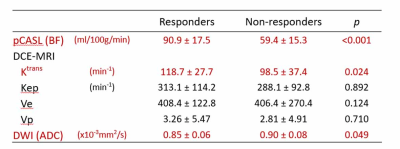
Twenty-five responders showed significantly higher BF (p < 0.001) and Ktrans (p = 0.024) than eleven non-responders (Fig. 1). ADC was significantly lower in responders than in non-responders (p = 0.049). For differentiation between responder and non-responder groups, the areas under the ROC curve of BF, Ktrans, and ADC was 0.909 (P < 0.001), 0.738 (P = 0.036), and 0.707 (P = 0.064), respectively. There was a significant positive correlation between BF and Ktrans (P = 0.006, ρ = 0.45).Discussion
Several studies have shown that Ktrans is the most important pharmacokinetic parameter of DCE-MRI with regard to predicting the tumor response to neoadjuvant chemoradiotherapy (NACRT) for rectal cancer 1,2). In our study, the responder group showed a higher Ktrans than the non-responder group. A high Ktrans indicates high permeability that is believed to make the tumor more accessible to CRT 2,3). No previous studies have shown the usefulness of pCASL for predicting tumor response to NAC or NACRT. In our study, BF was significantly higher in the responder group than in the non-responder group, and a significant positive correlation was obtained between BF and Ktrans. pCASL was at least as useful as DCE-MRI for response prediction after chemotherapy in locally advanced rectal cancer.Conclusion
pCASL may be a promising non-invasive, inexpensive alternative to DCE-MRI for predicting the treatment response of NAC for locally advanced rectal cancer.Acknowledgements
No acknowledgement found.References
1. Lim JS, Kim D, Baek S, et al. Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 2012; 22:1693-1700.
2. Tong T, Sun Y, Golub M, et al. Dynamic contrast-enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. Magn Reson Imaging 2015; 42:673-680.
3. Yeo D, Oh S, Jung C, et al. Correlation of dynamic contrast-enhanced MRI perfusion parameters with angiogenesis and biologic aggressiveness of rectal cancer: preliminary results. Magn Reson Imaging 2015; 41:474-480.