2318
The reduction in the contribution of a fast diffusing component for pancreatic malignancy detection – A preliminary study1Diagnostic Imaging Department, Sheba Medical Center, Ramat Gan, Israel, 2Department of Oncology, Sheba Medical Center, Ramat Gan, Israel, 3Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel, 4Life Sciences, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Early detection of pancreatic cancer is challenging but essential to improve its poor prognosis. This preliminary study utilized DT-MRI in pancreas in a clinical setting. DT-MRI was based on two pairs of b-values (0,500 s/mm2 and 100,500 s/mm2), such that the existence of a fast diffusing component was observed. We found that pancreatic tumors show similar values of parametric DTI measures (λ1 and MD-ADC) when using both b-value pairs, suggesting that the fast diffusing component is reduced compared with healthy or cystic pancreatic regions. We conclude that DT-MRI of the pancreas may assist in the detection of pancreatic tumors.
Introduction
Pancreatic cancer accounts for 3% of all cancers in the US and ~7% of all cancer deaths. Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and carries a poor prognosis of ~10% five-year survival rate. PDAC usually demonstrates clinical symptoms in an advanced stage of the disease in which the tumor is unresectable, therefore early detection is a pressing issue. This is especially important in high-risk populations, such as BRCA mutation carriers or patients with a family history of PDAC. Currently, there are no effective early detection strategies for PDAC1. Based on the structural and physiological features of the pancreas and the ability of diffusion tensor imaging (DTI) to track microstructural features, we have extended a preliminary study of the pancreas2 to a clinical setting. In this pilot study we searched for the differences in the contribution of a fast diffusing component within the DTI parametric measures among four groups; normal tissue in healthy patients and in cancer patients, intraductal papillary mucinous neoplasm (IPMN) tissue and PDAC tumors. We aimed at exploring the potential of pancreas DT-MRI in PDAC detection.Methods and Analysis
6 healthy controls, 9 PDAC patients and 9 IPMN patients were scanned on a 3T whole body Philips Ingenia MRI scanner using a 32-channel radio body coil with a full clinical protocol (T1 weighted, T2 weighted, DCE, MRCP, multi b-values DWI). In our institution, DTI is added to the clinical protocol for pancreatic cancer diagnosed patients or high risk patients. DTI is based on spin echo with TR/TE = 3099/74 ms, compressed SENSE factor 2, 32 diffusion directions, b-values of 0, 100 and 500 s/mm2, fat suppression, respiratory triggering, spatial resolution of 3x3x3 mm2 and scan time of approximately 4 minutes. The diffusion tensor parameters were calculated separately for two pairs of b-values: 0, 500 s/mm2 and 100,500 s/mm2 using a proprietary software program described earlier2. For b=100 s/mm2 the signal intensity was averaged over the 32 diffusion directions. In the PDAC patients, tumors were manually marked by an expert radiologist with more than 30 years of experience in body MRI on a T2-weighted image. Also, when possible, a healthy region of the pancreatic tissue was marked. In the healthy controls and the IPMN patients, head and tail regions of the pancreas were marked. The ROIs were then copied to the DTI images (which were scanned using the same slice thickness and field of view as the T2-weighted images). Average values were calculated in all ROIs and in all DTI derived parametric maps using both pairs of b-values. Then, in MD-ADC and λ1, for each ROI, the ratio between average values derived from the analysis of b-value pairs 0, 500 s/mm2 and 100,500 s/mm2 was computed. Ratio values were then averaged per group and statistical analysis (t-test) was performed between the groups.Results and Discussion
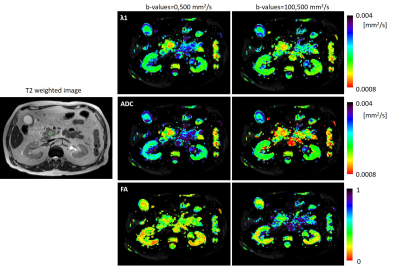
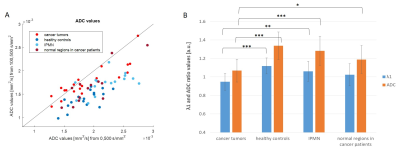
Figure 1 shows T2-weighted and DTI derived MD-ADC, λ1 and FA maps of a representative PDAC patient. The tumor is present in the pancreas head and is detected on both the λ1 and MD-ADC maps as a red region surrounded by a greenish healthy tissue. It is marked on the T2-weighted image. The tumor is more prominent when using b-values of 0,500 mm2/s. The FA maps do not show a clear difference between the tumor and its surrounding. Figure 2A shows the correlation between the average ROIs’ MD-ADC values, derived from DTI using both pairs of b-values in four groups of data as described above. Most data points show smaller values when using b-values of 100,500 s/mm2 compared with b-values of 0,500 s/mm2, suggesting that a fast diffusing component is present, which is most likely attributed to intra-voxel-incoherent-motion (IVIM)3. This is not true for most of the data points that represent the tumor ROIs (bright red), which are close to the diagonal line (in MD-ADC, Figure 2), there the values are similar when using each of the pairs of b-values. The fast diffusing component is reduced in these cases, which correlates with the lower microvascular fraction in PDAC as compared to normal pancreatic tissue, as reported previously by Lemke et al3. Figure 2B shows the average ratio values in λ1 and MD-ADC from all ROIs in each of the four groups. The cancerous tumor regions show a ratio which is close to 1, while the healthy control regions show the largest deviation from 1. This is true for both λ1 and MD-ADC. IPMN regions show intermediate λ1 and MD-ADC ratio values. The ratio values of the cancerous tumors are statistically different from all other three groups in both λ1 and MD-ADC, except for the case of the cancerous tumors and the normal regions in those same patients when using λ1.Conclusions
The results of this preliminary study shows the feasibility of pancreas DT-MRI in a clinical setting. Our findings suggest that an observation of the degree of contribution of a fast diffusing component to the values of the DTI parametric measures may assist in the identification of pancreatic malignancy, as a complementary method to the usual clinical scan.Acknowledgements
No acknowledgement found.References
1. Singhi AD, Koay EJ, Chari ST, et al. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology 2019;156:2024-2040.
2. Nissan N, Golan T, Furman-Haran E, et al. Diffusion Tensor Magnetic Resonance Imaging of the Pancreas. PLoS ONE 2014;9:e115783.
3. Lemke A, Laun FB, Klauss M, et al. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009;44:769-75.
Figures