Mark Smith1, Harry Hu2, Ram Krishnamurthy1, John Pitts3, and Mai-Lan Ho1
1Nationwide Children's Hospital, Columbus, OH, United States, 2Hyperfine, Dublin, OH, United States, 3Hyperfine, Cleveland, OH, United States
Synopsis
In 2020, a portable 64mT ultra-low field MRI system designed
for point of care bedside use received 510K clearance (Hyperfine, Guilford,
CT). Our pediatric institution
(Nationwide Children’s Hospital)
has acquired one of these systems to meet neuroimaging needs for critically ill
NICU or PICU patients who cannot tolerate transport to the MRI department. Prior to scanning patients, safety testing
for displacement and heating was conducted on monitoring hardware that will be
connected to the patient during the bedside MRI. The monitoring hardware was found safe to
stay connected to the patient during the bedside MRI.
Purpose
Over the last several decades, Magnetic
Resonance Imaging (MRI) has emerged as a powerful imaging modality. The applications of MRI continue to expand
rapidly, but the cost, infrastructure and safety requirements of MRI prohibits
its utility in certain patient populations due to logistics, critical illness, contraindicated
implanted devices and monitoring equipment.
Recently, a portable 64mT ultra-low field MRI system received 510K
clearance (Hyperfine, Guilford, CT). This is a point of care (POC) system
designed for bedside use (Figure 1), affording neuroimaging to patients
in circumstances where conventional MRI is not possible. Our pediatric institution (Nationwide
Children’s Hospital) recently acquired one of these systems,
with the intent to meet neuroimaging needs for critically ill NICU or PICU
patients who cannot tolerate transport to the MRI department. Most of these patients are on continuous
monitoring and / or life support, requiring that electronic equipment and
associated hardware remain operational and connected to the patient during the
bedside MRI exam. Prior to scanning
patients with the portable MRI, safety testing should be conducted on
monitoring hardware likely to be in place during the bedside MRI, especially
hardware that is MR unsafe for conventional high-field MRI. FDA 510K clearance of the Hyperfine system did
not include compatibility of any of the tested equipment in this abstract.Methods
Patient monitoring hardware commonly used
in our NICU and PICU was identified.
This included five types of electrodes (Kendall 1041PTS and 1042PTS
neonatal electrodes, Kendall L36-L6 cloth limb band electrodes, Kendall
neonatal-pediatric monitoring electrodes with aloe, and 3M Red Dot neonatal ECG
monitoring electrodes), a Nellcor neonatal-adult SpO2 sensor and a MEAS 4400
series esophageal temperature probe.
Each of these underwent safety tests for displacement and heating within
the portable MRI scanner. Wick
traditional hemoclips, an Abbott ECMO CentriMag pump (Figure 2) and six
ECMO catheters (Medtronic Biomedicus 17 Fr. 50 cm. femoral-venous, 17 Fr. 18 cm
arterial, 14 Fr. 12 cm arterial, 14 Fr. 12 cm venous, DLP 14 Fr. “bullet tip”, and
Getinge Avalon 16 Fr. bi-caval dual lumen) were also subject to the same
tests. Displacement testing simply
consisted of placing each hardware item on the scanner magnet plate and
observing attractive forces. Testing for
heating consisted of placing each item in a tissue mimicking agar gel tub within
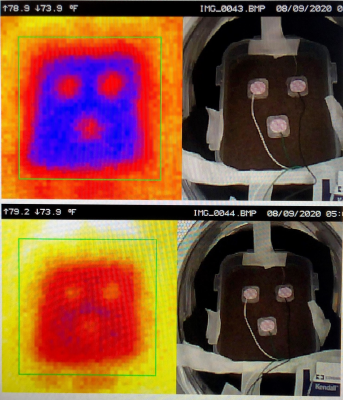
the scanner, and taking photos and thermal images using a hand held thermal camera
(Ideal Industries, Model # 61-844) to record temperature changes before and
after 30 minutes of continuous scanning (Figure 3). The 30 min scan
consisted of typical spin echo pulse sequences (T1, T2, T2 FLAIR, DWI). Additional thermal images were taken with the
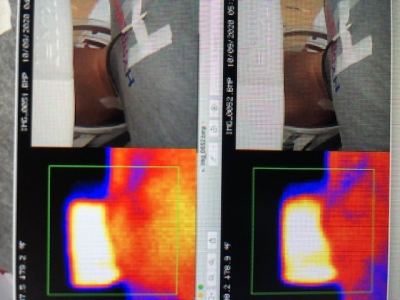
ECMO catheters lying on the bare neck of a volunteer before and after a 40 min
scan conducted under IRB approval (Figure 4). All testing was done and data evaluated by a
MRSO / MR physicist.Results
All five types of electrodes, the SpO2
sensor, temperature probe and hemoclips were non-ferrous and were not subject
to translational forces nor displacement, and pre / post scan temperature changes
were ≤ 2o
C. The ECMO CentriMag pump housed a
magnet in the impeller wheel, resulting in displacement of the impeller, and
translational force exerted on the pump at a 50 cm. distance from the edge of
the scanner. Because of this, a heating
test was not performed on the pump. All
six ECMO catheters exhibited weak displacement within the scanner due to
ferrous coiling within catheter tubing. Pre
/ post scan temperature changes were ≤ 2o C for all six ECMO catheters when placed
on the gel tub. There was a pre / post
scan 4o C temperature increase in the Medtronic Biomedicus DLP 14
Fr. “bullet tip” catheter
when placed on the bare neck of the volunteer, but this may have been due to
inconsistency in positioning of the thermal camera. Pre / post scan temperature changes in the
rest of the ECMO catheters when placed on the bare neck of the volunteer were ≤ 2o C, and went unnoticed by the volunteer.Conclusion
Our initial tests on this portable ULF
MRI scanner revealed no safety concerns due to displacement or heating, apart
from the ECMO CentriMag pump. In a patient setting, positioning the CentriMag
pump and the integrated ECMO system apparatus at least one meter in distance
from the scanner edge is recommended. All
the hardware tested above is MR unsafe or conditional at 1.5T and 3T. Other equipment that we have not tested but we
consider potentially unsafe include Camino ICP monitors, LVAD, cardiac pacing
wires and the Pleuraflow Chest tube with magnetic strip..Acknowledgements
No acknowledgement found.References
1.
Sheth KN, et al.
Assessment of Brain Injury Using Portable Low Field MRI at the Bedside of
Critically Ill Patients. JAMA Neurology
2020; E1-7. doi:10.1001/jamaneurol.2020.3263.