2310
Is a Local Tx Coil sufficient for Guidewire Safety in MRI?1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 3Department of Electrical Engineering, Stanford, Stanford, CA, United States
Synopsis
Imaging guidewires in MRI is known to have heating hazards associated. In this work we show initial data that supports the use of local Tx coils over whole body coils for the safe imaging of standard guidewires with MRI. This was tested in vitro using parallel transmit (PTx) techniques to generate a scenario for a proper comparison between the two coil types. It was found that a local Tx coil produces less heating at the guidewire tip, than a body coil under comparable conditions.
Introduction
MRI guided cardiac interventions pose inherent challenges in relation to use of metallic guidewires, particularly as a result of risk of radio-frequency (RF) induced heating at the distal tip (1). Some studies have used parallel transmit (PTx) technology to actively control the induced currents on guidewires (2,3). A simpler, more common approach is to mitigate heating risk by reducing the incident RF (4,5), or working at lower field which induces less coupling (6). Standard clinical scanners generally feature body sized transmit (Tx) coils, and so, much of the work has focused on that kind of excitation coil. However, in prior work done using an in vivo sheep model with a local PTx coil array, we observed that even worst-case conditions lead only to temperature increases of a few degrees (7). Hence, we hypothesize that local Tx coils inherently result in less heating at the guidewire tip than standard body-coils. The safety benefits of local Tx coils for deep brain stimulators has been reported before (8), but so far this hasn’t been extended to cardiac interventions, that are the focus of our work. A proper comparison would require that the coupling between guidewire and Tx coils be comparable in both coil configurations. This condition is difficult to predict directly from geometry alone, but with the added degrees of freedom afforded by a PTx system, such worse-case conditions can be determined for any geometry. In this work we looked at guidewire tip heating using either a PTx body coil or PTx local coil, directly comparing heating under worst-case conditions.Methods
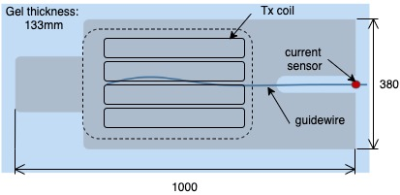
All measurements were performed on a 3T Philips Achieva. A built-in 8-channel TEM transmit-receive body coil (9)was compared to a local Tx coil array comprised of 4 anterior and 4 posterior loops (Rapid Biomedical, Germany). A 1m long nitinol guidewire (Terumo Corporation, Japan) stripped back to bare wire over a 2cm region at its distal tip, was placed in a large anthropomorphic phantom (figure 1) filled with 32 Litres of poly acrylic acid gel (10). The guidewire length external to the phantom was instrumented by an optically coupled toroidal current sensor and was connected to the scanner’s spectrometer for digitization. The signals from current sensor were used to determine worst-case coupling between the guide wire and the PTx coil under test, as described in (11). Temperature at the distal end of the wire was monitored using a fibre-optic temperature probe (LumaSense Technologies, Inc. USA). Heating was measured during a high specific absorption rate (SAR) balanced steady state free precession sequence played out while in the worst-case coupling mode. Power levels were adjusted to achieve similar excitation RF fields (B1+) from both PTx coils, accounting for differences in matching and coil efficiencies. Volumetric actual flip angle imaging (12) was used to measure B1+ using the quad (i.e. circularly polarized) mode of each coil, using the same average power level subsequently used for heating tests.Results
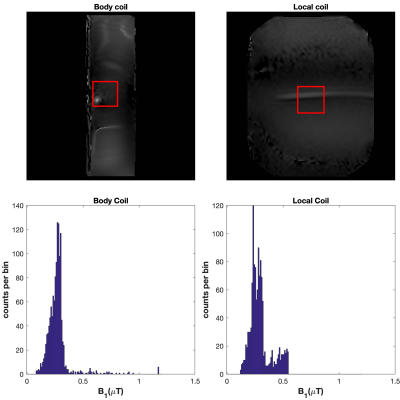
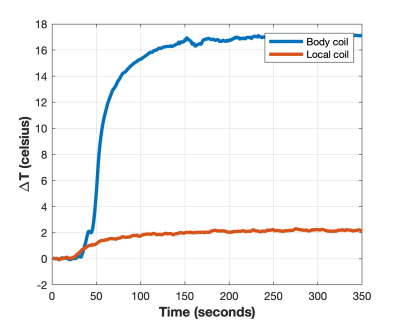
The average quad-mode B1+ fields for the body and local coil were 0.27µT and 0.29µT respectively; B1+ maps and histograms for a region of interest at the centre is shown in figure 2. This demonstrates that the appropriate power level was selected to match the imaging performance of both arrays. The temperature profiles of each coil type while imaging in the worst-case scenario at the same power level is shown figure 3. The body coil produced a temperature increase of 17ºC, while the local coil only produced an increase of 2ºC, under the same conditions.Discussion
In this work we explored the worst-case heating of a guidewire, simulating a cardiac intervention, using a PTx body-coil compared with a PTx local coil, and observed that the former results in much greater heating for equivalent imaging conditions (i.e. matched B1+). PTx coils were used for this comparison so that a worst-case coupling condition could be achieved by using measured current sensor data, giving a more robustly obtainable worst case operating condition. This was motivated by previous experience from experiments on an in vivo sheep model with a local surface array transmit coil, in which only 0.25°C of heating was observed on average (increasing to 3°C ex vivowith the absence of blood-flow related cooling) (7). A possible explanation may be that a local coil produces EM fields over a smaller portion of the wire compared to the body coil. This is acceptable for cardiac imaging where the imaging field of view is usually restricted to the heart by the presence of a local receiver. Another study (8) of local head coils compared with body-coil excitation found similar trends for imaging of patients with neurostimulation devices.Conclusion
We have demonstrated preliminary data that suggests local Tx coils may be an intrinsically safer option for interventional cardiac MRI than large body coils. Future work will seek to explore this observation in a broader context of simulation and experiment.Acknowledgements
Funding for this project is provided by the Medical Research Council (MRC) developmental pathway funding (MR/N027949), the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z), and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Razavi R, Hill DLG, Keevil SF, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet 2003;362:1877–1882.
2. Godinez F, Hajnal J V, Malik SJ. Auxiliary PTx system for active control of induced RF currents in conductive guidewires. In: Proc. Intl. Soc. Mag. Reson. Med. 27. ; 2019. p. 0267.
3. Teixeira JN, Godinez F, Malik S, Hajnal J V. Exploring the impact of nulling currents on a cardiac guidewire in reducing worst case SAR at 1 . 5T. In: Proc. Intl. Soc. Mag. Reson. Med. Paris France: ISMRM; 2018. p. 0299.
4. Campbell-Washburn AE, Tavallaei MA, Pop M, Grant EK, Chubb H, Rhode K, Wright GA. Real-time MRI guidance of cardiac interventions. J. Magn. Reson. Imaging doi: 10.1002/jmri.25749.
5. Campbell-Washburn AE, Rogers T, Basar B, Sonmez M, Kocaturk O, Lederman RJ, Hansen MS, Faranesh AZ. Positive contrast spiral imaging for visualization of commercial nitinol guidewires with reduced heating. J. Cardiovasc. Magn. Reson. 2015;17:114.
6. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019;293:384–393.
7. Godinez F, Tomi-tricot R, Delcey M, Lykowsky G, Williams SE, Quesson B, Hajnal J V. Dynamic control of RF currents in conductive guidewires with an auxiliary PTx system : First in vivo experience in sheep. In: Proc. Intl. Soc. Mag. Reson. Med. 28. Paris France: ISMRM; 2020. p. 0110.
8. Rezai AR, Finelli D, Nyenhuis JA, Hrdlicka G, Tkach J, Sharan A, Rugieri P, Stypulkowski PH, Shellock FG. Neurostimulation systems for deep brain stimulation: In vitro evaluation of magnetic resonance imaging-related heating at 1.5 Tesla. J. Magn. Reson. Imaging 2002;15:241–250.
9. Vernickel P, Röschmann P, Findeklee C, Lüdeke K-M, Leussler C, Overweg J, Katscher U, Grässlin I, Schünemann K. Eight-channel transmit/receive body MRI coil at 3T. Magn. Reson. Med. 2007;58:381–389.
10. ASTM standard F 2182-2002a. Standard test method for measurement of radio frequency induced heating near passive implants during magnetic resonance imaging. ASTM Int. doi: 10.1520/F2182-11A.1.7.
11. Godinez F, Scott G, Padormo F, Hajnal J V., Malik SJ. Safe guidewire visualization using the modes of a PTx transmit array MR system. Magn. Reson. Med. 2020;83:2343–2355.12. Nehrke K. On the steady-state properties of actual flip angle imaging (AFI). Magn. Reson. Med. 2009;61:84–92.