2297
An anthropomorphic phantom for deep brain stimulation MRI safety investigations1Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 2Electrical and Computer Engineering, McMaster University, Hamilton, ON, Canada, 3Division of Neurosurgery, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 4Harquail Centre for Neuromodulation, Sunnybrook Research Institute, Toronto, ON, Canada, 5Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Magnetic resonance imaging of deep brain stimulation (DBS) patients remains a safety concern at higher magnetic fields strengths. Phantoms play an important role in validating and estimating patient safety conditions. However, conventional phantoms are typically simple homogeneous structures that limit the ability to replicate DBS surgical implant procedures. In this work, a new phantom structure is proposed with more human-like realism and compartments to enable improved replication of DBS device placement geometries. Preliminary radiofrequency heating results demonstrate significant differences in temperature elevations when the phantom is assembled in a heterogeneous and homogeneous configuration.
Introduction
Magnetic resonance imaging (MRI) of deep brain stimulation (DBS) patients remains a safety concern at higher magnetic field strengths. The current practice for 1.5 T MRI recommends strict imaging guidelines for approved medical devices [1-2]. Without approved guidelines for imaging at 3 T and above, MRI researchers continue to investigate potential solutions in efforts to achieve safe unrestricted imaging conditions for DBS patients in the future [3]. Researchers rely heavily on electromagnetic simulation tools to develop potential methodologies to address this problem. Anatomical models used in these simulations have advanced to include multiple tissue compartments and realistic human features [4]. Unique implanted DBS lead(s) geometries have also been reported to impact electromagnetic behaviour [5]. It appears necessary to continue to investigate the impact of DBS implant geometries. However, standard phantoms are typically simple homogeneous structures that provide a limited approximation of DBS device placements [6]. It is thus logical to improve phantom structures that incorporate human-like characteristics. The present work utilizes fast 3-dimensional printing technology to construct a new anthropomorphic phantom for DBS patient safety studies. The methods and results summarize published work by Yang et al. for two phantom configurations: (1) a heterogeneous setup and (2) homogeneous setup. An MRI experiment involving another patient-derived DBS trajectory is also in progress, to study the potential for localized temperature increases where the DBS lead is bundled and subsequently enters the brain through the skull.Methods
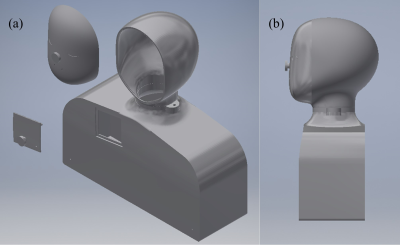
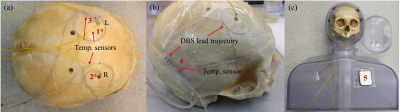
The computer-aided design model was developed in Inventor 2019 (Autodesk, San Rafael, CA, USA) with human features such as a chin and neck with reasonably realistic curvature, as shown in Figure 1 [7]. A human skull with targeted blur holes enabled a realistic DBS implant geometry to be tested, as shown in Figure 2 [7]. Five fibre-optic temperature sensors (Neoptix, Quebec City, QB, CAN and Opsens, Quebec City, QB, CAN) were placed at strategic locations to measure temperature elevations, labelled in Figure 2. Sensors labelled 1* and 2* were sutured at the tip of the corresponding DBS lead (3387, Medtronics, Minneapolis, MN, USA) and implanted to the approximate location of the thalamus. The remaining sensors labelled 3 to 5 were taped at the spiral trajectory near the left blur hole, at the DBS lead extension and onto the deep surface of the neurostimulator (Activa PC, Medtronics, Minneapolis, MN, USA), respectively. Turbo spin-echo (TSE) imaging experiments were conducted on a 3T MRI system (Magnetom Prisma, Siemens, Erlangen, DEU) that measured temperature elevations at the locations described above for DBS device on and off states in two phantom configurations. Configuration 1 was a heterogeneous configuration, with a grey matter-mimicking solution (conductivity of approximately 0.69 S/m and relative permittivity of approximately 67) filling the human skull and the ASTM body average solution (conductivity of approximately 0.47 S/m and relative permittivity of approximately 80) filling the remaining structure. Configuration 2 was a homogeneous configuration that filled the structure with only the ASTM body average solution and routed the two DBS leads along the inner wall to the top of the head, then towards the centre approximating the location of the thalamus [6].Results
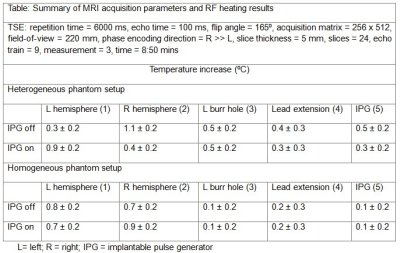
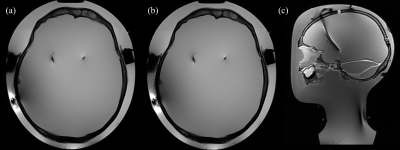
The table in Figure 3 summarizes the MRI experimental results [7]. The maximum temperature rise was 1.1 ± 0.2⁰C (DBS device off) for the heterogeneous setup and 0.9 ± 0.2⁰C (DBS device on) for the homogeneous setup. Figure 4 illustrates the reconstructed phantom images in transverse and sagittal views for two TSE protocols (TR / TE = 6000 ms / 100 ms, flip angle = 165°, time = 8:50 min and TR / TE = 516 ms / 6.7 ms, flip angle = 150°, time = 3:45 mins, respectively) [7].Discussion and Conclusion
The experimental results showed significant temperature elevation differences for the two phantom setups, indicating that complex phantom structures may be beneficial when investigating DBS patient safety. In conclusion, a promising phantom is presented along with preliminary results indicating improved safety estimation may be achievable with more advanced phantom structures. Although conceptually logical, future work will involve electromagnetic simulation to confirm the effectiveness of the anthropomorphic phantom, with some preliminary studies on the impact of the skull layer already in progress.Acknowledgements
No acknowledgement found.References
[1] Medtronic Inc. MRI guidelines for Medtronic deep brain stimulation systems 2015.
[2] Medicines and Healthcare Products Regulatory Agency. Safety guidelines for magnetic resonance imaging equipment in clinical use 2014.
[3] McElcheran et al., Parallel radiofrequency transmission at 3 Tesla to improve safety in bilateral implanted wires in a heterogeneous model. Magn Reson Med. 2017.
[4] Guerin et al., Parallel transmission to reduce absorbed power around deep brain stimulation devices in MRI: impact of number and arrangement of transmit channels. Magn Reson Med. 2020.
[5] Golestanirad et al., RF-induced heating in tissue near bilateral DBS implants during MRI at 1.5 T and 3T: the role of surgical lead management. NeuroImage. 2019.
[6] Boutet et al., 3-Tesla MRI of deep brain stimulation patients: safety assessment of coils and pulse sequences, J. Neurosurg. 2019.
[7] Yang et al., “Technical Note: An anthropomorphic phantom with implanted neurostimulator for investigation of MRI safety.” Med. Phys. 2020.
Figures