2292
MRI in patients with a prosthetic heart valve, annuloplasty ring or mitra clip; Guideline in the Netherlands1Radiology and Nuclear Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, 2Catharina Hospital, Eindhoven, Netherlands, 3Radiology and Nuclear Medicine, University Medical Centre Utrecht, Utrecht, Netherlands, 4Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands, 5Knowledge Institute Medical Specialists, Utrecht, Netherlands, 6Radiology and Nuclear Medicine, Amsterdam UMC location AMC, Amsterdam, Netherlands, 7Radiology, LUMC, Leiden, Netherlands, 8Maxima Medical Centre, Eindhoven, Netherlands, 9CJ Gorter Center for High Field MRI, LUMC, Leiden, Netherlands, 10Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, 11Radiology, UMCG, Groningen, Netherlands, 12Imaging, Radboud UMC, Nijmegen, Netherlands
Synopsis
Can a patient with a prosthetic heart valve, annuloplasty ring or a mitra clip undergo an MRI examination? The Dutch Association of Medical Specialists (FMS) wrote a guideline, using searches of guidelines, literature, implant databases, and incident databases. The recommendation is: scan the patient with a prosthetic heart valve, annuloplasty ring or mitra clip with an 1.5T or 3T whole body MRI system with a horizontal closed bore superconducting magnet without further restrictions.
Introduction
There is a wide variety of types of heart valve prostheses and annuloplasty rings for which there are various specific conditions for performing an MRI. Many of these manufacturer-set conditions are conservative such that they may impair patients diagnosis. Additionally, in some cases the type of heart valve or the safety profile of the heart valve is unknown. Hospitals vary in their policies regarding screening and use of MRI in these patients. This is the case for all type of MRI examinations and not only for MRI of the heart. Therefore the Dutch Association of Medical Specialists (called FMS) has written a guideline on the clinical question: can a patient with a prosthetic heart valve, annuloplasty ring or mitra clip undergo an MRI examination in a 1.5T or 3T whole body MRI systems with a horizontal closed bore superconducting magnet?Methods
The guideline has been developed conforming to the standard within the FMS as described in Guidelines for Medical Specialists 2.0 1, based on the AGREE II instrument 2. The guideline, initiated by Society for Medical Physics of the Netherlands (NVKF), was written by a working group together with participants from the Dutch Society for Medical Imaging and Radiotherapy (NVMBR), the Radiological Society of the Netherlands (NVvR), the Netherlands Society of Cardiology (NVvC) and advisors from the Knowledge Institute of the FMS. A series of searches were performed: guideline search, literature search, implant search on MRI safety.com and Magresource 3, and a search on implant incident databases of FDA, the Health Inspection Service in the Netherlands, and the 'Implant' and 'Event' database of the International Consortium of Investigative Journalists. In order to come to a recommendation, in addition to (the quality of) the scientific evidence, other aspects are important and were taken into account, such as the expertise of the working group members, cost/benefits, availability of facilities and organization of healthcare. These aspects were discussed in the Considerations. The concept guideline was subjected to commentaries by the involved Dutch (scientific) associations, agencies and (patient) organizations. The final version was authorized by the end of 2019 by the FMS.Literature Review
Literature was searched to answer the question “Can a patient with a prosthetic heart valve or annuloplasty ring undergo MRI examination?”. The conclusion is that during MRI examinations at 3T or below, no interactions between a prosthetic heart valve and the magnetic fields and radio frequency waves caused by the MRI scanner have been detected that could be harmful to the patient 4-14. Patients with degenerative valve disease are unlikely to have a greater risk of valve loosening as a result of MRI 15. Up to 1.5T, there is some evidence, although limited, that MRI does not cause cardiac dysrhythmia in patients with a prosthetic heart valve 8,16.Considerations
In none of the incident databases reports were found to be relevant for this guideline, neither we identified reported incidents in the literature. In the Netherlands many patients with prosthetic heart valves, annuloplasty rings or mitra clips have been scanned over the years at 1.5 and 3T systems without further limitations, in which the benefit of an MRI exam outweighed the potential risks.Risks assessment for the heart valve implants was performed based on the standard risks for metallic implants in the MRI:
- Risk of displacement and rotation of the implant due to the presence of the static magnetic field and the spatial gradient of this field. Forces on the few slightly ferromagnetic implants are small compared to physiological forces 14. Limitations set on spatial gradients by the manufacturers (Figure 1) were given by limitation of the test situation, and not so much by observed complications.
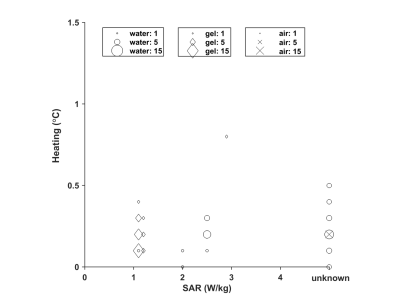
- Risk of implant heating due to interaction with RF field. Manufactures give SAR limitations, see Figure 1. Heating is measured by in vitro tests, which typically report heating instead of additional heating (Figure 2). Additional heating as reported in the literature is less (Figure 3). More importantly, these implants are effectively cooled by the blood flow which is not taken into account by the risk assessment of the manufacturers 17.
- Risk of vibration or induction of currents by the oscillating magnetic field gradients applied for the spatial encoding of the MRI signal. This is considered insignificant due to the small surface of these implants.
- Artifact in the MRI image. This is typically limited to less than 1 cm around the implant.
- Risk of forces due to the Lenz effect during rapid movement of conductive implants in the static magnetic field of the MRI scanner. This effect is suggested to limit valve motion 18,19. With reasons, see whole guideline, we think that this effect is insignificant in clinical practice.
- Risk of Implant Disruption. The only dysfunction could be caused by the above mentioned Lenz effect.
Recommendation
A patient with a prosthetic heart valve, annuloplasty ring or mitra clip can be scanned without further restrictions with an 1.5T or 3T whole body MRI system with a horizontal closed bore superconducting magnet.The whole guideline can be found in the FMS guideline database 20. An English translation will appear early 2021. The guideline will be reviewed in five years, or earlier when necessary.
Acknowledgements
The guideline development was financed by the Quality Funds for Medical Specialists (Stichting Kwaliteitsgelden Medisch Specialisten: SKMS). We thank Diana P Gutierrez for secretary support of the guideline project, and Evert J Vonken and Albert van der Zwan for participating in the working group.References
1. Adviescommissie Richtlijnen van de Raad Kwaliteit. Medisch Specialistische Richtlijnen 2.0; 2012. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
2. Brouwers M, Kho ME, Browman GP, et al. on behalf of the AGREE Next Steps Consortium: AGREE II: Advancing guideline development, reporting and evaluation in healthcare. CMAJ; 2010; www.agreetrust.org.
3. MagResource, MR:comp GmbH, Gelsenkirchen, Germany; www.MagResource.com
4. Edwards MB, Phil M, Ordidge RJ, et al. Translational and rotational forces on heart valve prostheses subjected ex vivo to a 4.7-T MR System. J Magn Reson Imaging 2002;16:653-659.
5. Edwards MB, Phil M, Taylor KM, Shellock FG. Prosthetic heart valves: Evaluation of magnetic field interactions, heating, and artifacts at 1.5 T. J Magn Reson Imaging 2000;12:363-369.
6. Edwards MB, Phil M, Ordidge RJ, et al. Assessment of magnetic field (4.7 T) induced forces on prosthetic heart valves and annuloplasty rings. J Magn Reson Imaging 2005;22:311-317.
7. Pruefer D, Kalden P, Schreiber W, et al. In vitro investigation of prosthetic heart valves in magnetic resonance imaging: Evaluation of potential hazards. J Heart Valve Dis. 2001; 10:410-4.
8. Randall PA, Kohman LJ, Scalzetti EM, et al. Magnetic Resonance Imaging of prosthetic cardiac valves in vitro and in vivo. Am J Cardiol. 1988;62:973-976.
9. Saeedi M, Thomas A, Shellock FG. Evaluation of MRI issues at 3-Tesla for a transcatheter aortic valve replacement (TAVR) bioprosthesis. Magn Reson Imaging 2015;33:497-501.
10. Shellock FG. Prosthetic heart valves and annuloplasty rings: Assessment of magnetic field interactions, heating, and artifacts at 1.5 Tesla. J Cardiovasc Magn Reson. 2001;3,317-324.
11. Shellock FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-Tesla MR system. J Magn Reson Imaging 2002;16:721-32.
12. Shellock FG and Crues JV. High-field-strength MR imaging and metallic biomedical implants: An ex vivo evaluation of deflection forces. Am J Roentgenol. 1988;151:389-92.
13. Shellock FG and Morisoli SM. Ex vivo evaluation of ferromagnetism, heating, and artifacts produced by heart valve prostheses exposed to a 1.5-T MR system. J Magn Reson Imaging 1994;4:756-758.
14. Soulen RL, Budinger TF, Higgins CB Magnetic resonance imaging of prosthetic heart valves. Radiology 1985;154:705-707.
15. Edwards MB, Draper ERC, Phil M, et al. Young I.R. Mechanical testing of human cardiac tissue: some implications for MRI safety. J Cardiovasc Magn Reson. 2005;7:835–840.
16. Hartnell GG, Spence L, Hughes LA, et al. Safety of MR Imaging in Patients Who Have Retained Metallic Materials After Cardiac Surgery. Am J Roentgenol. 1997;168:1157-9.
17. Stijnman RS, van Nierop BJ, et al. Assessment of the alleviating impact of perfusion on RF-induced heating due to artificial cardiac valves. Annual Meeting ISMRM 2019, 4154.
18. Condon B and Hadley DM. Potential MR hazard to patients with metallic heart valves: the Lenz effect. J Magn Reson Imaging 2000;12:171-176.
19. Golestanirad L, Dlala E, Wright G, et al. Comprehensive analysis of Lenz effect on the artificial heart valves during magnetic resonance imaging. Prog Electromagn Research 2012:128,1-17.
20. Guideline database, Federation Medical Specialists, the Netherlands. https://richtlijnendatabase.nl/richtlijn/gebruik_mri_bij_patienten_met_implantaten/startpagina_-_mri_bij_patienten_met_implantaten.html
Figures
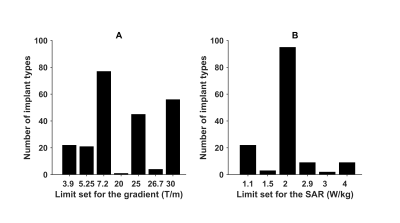
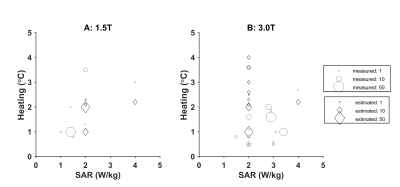
Figure 2: Manufacturer estimated (Δ) and measured maximum (o) RF heating in an implanted gel phantom at 1.5 T (A) and 3.0 T (B). The size of the symbols is proportional to the number of values reported for different types of valves/rings.