2285
MRI in patients with a cerebral aneurysm clip; Guideline in the Netherlands1Radiology and Nuclear Medicine, Amsterdam UMC, Vrije Universiteit, Amsterdam, Netherlands, 2Radiology and Nuclear Medicine, Amsterdam UMC location AMC, Amsterdam, Netherlands, 3, Neurology and Neurosurgery, UMC Utrecht Brain Center, Utrecht, Netherlands, 4Maxima Medical Centre, Eindhoven, Netherlands, 5Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands, 6Radiology, LUMC, Leiden, Netherlands, 7Imaging, Radboud UMC, Nijmegen, Netherlands, 8Catharina Hospital, Eindhoven, Netherlands, 9Radiology and Nuclear Medicine, University Medical Centre Utrecht, Utrecht, Netherlands, 10CJ Gorter Center for High Field MRI, LUMC, Leiden, Netherlands, 11Radiology, UMCG, Groningen, Netherlands, 12Knowledge Institute Medical Specialists, Utrecht, Netherlands, 13Radiology and Nuclear Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
Synopsis
The Dutch Association of Medical Specialists wrote a guideline for MR safety experts for an MRI examination in a patient with a cerebral aneurysm clip for known and unknown clip types. Risk stratification for unknown clip types was based on a survey in the Netherlands that identified time frames and locations of ferromagnetic clip use.
Introduction
Although in the Netherlands nowadays only MR-conditional or MR-safe clips are utilized, ferromagnetic type clips have been used until late in the last century. As this treatment is given to patients in different age groups including young adults, patients with an aneurysm clip, implanted in a period when ferromagnetic types were still in use can present themselves for MRI for decades to come.If the type of cerebral aneurysm clip is known, it is possible to determine whether the patient can safely undergo an MRI examination. However, in the past, patients have received clips that are an absolute contraindication for MRI, so caution is advised when the type of clip is unknown or it is not possible to determine with certainty what clip was implanted. In daily practice in the Netherlands, it is often unknown which the type of clip was implanted, as it is also in other countries 1,2. Therefore, the Dutch Association of Medical Specialists (called FMS) has written a guideline for MR safety experts to develop a local policy for safely performing an MRI examinations in patients with a cerebral aneurysm clip, including strategies for known and unknown clip types, for 1.5T or 3T whole body MRI systems with a horizontal closed bore superconducting magnet.
Methods
The guideline has been developed conforming to the standard within the FMS as described in Guidelines for Medical Specialists 2.0 3, based on the AGREE II instrument 4. The guideline, initiated by Society for Medical Physics of the Netherlands (NVKF), was written by a working group together with participants from the Dutch Society for Medical Imaging and Radiotherapy (NVMBR), Radiological Society of the Netherlands (NVvR), Netherlands Society for Neurosurgery (NVvN) and advisors from the Knowledge Institute of the FMS. A series of searches for evidence were performed in existing guidelines, literature, MRI safety.com and MagResource 5, and implant incident databases of FDA, the Health Inspection Service in the Netherlands, and the 'Implant' and 'Event' database of the International Consortium of Investigative Journalists.In addition to the scientific evidence, other aspects are important and were taken into account, such as the expertise of the working group members, availability of facilities and organization of healthcare. The concept guideline was subjected to commentaries by the involved Dutch (scientific) associations, agencies and (patient) organizations. The final version was authorized in 2019 by the FMS.
Literature Review
Literature was searched to answer the question “what is the chance of a negative outcome for an MRI in a patient with a ferromagnetic clip?”. There is a chance that a patient with a ferromagnetic aneurysm clip can undergo the MRI examination without complications, but there is also a realistic chance that the examination turns out to be fatal: estimating the individual risk to the patient is complicated and depends on the condition of the vessel wall on which the aneurysm clip is placed 6-9. Non-ferromagnetic clips appear to be safe in the MRI environment 10-13.On the question regarding the period of implantation of contraindicated clips in the Netherlands, based upon studies mainly from the United States, we inferred the chance that a cerebral clip is ferromagnetic is high for implantation before the mid-80s and low after the mid-90s. It should be noted that the implantation of ferromagnetic clips continued for some time after the production of these clips ended 8,14,15.
Considerations
A list of MR unsafe clips was made (Table 1). In none of the incident databases we found reports to be relevant for this guideline. However, one incident was identified in the literature 16, and recently one was reported 17. Because no information was found on the timeframe when implantation of contraindicated clips took place in the Netherlands, a survey was conducted, see Table 2 and 3.Risks assessment for the clips was performed based on the standard risks for metallic implants in the MRI:
- Risk of displacement and rotation of the implant due to the presence of the static magnetic field and the spatial gradient of this field. This is the main risk for ferromagnetic clips. For non-ferromagnetic models the risk is negligible. For an unknown type of clip the working group estimated the risk (Table 4).
- Risk of implant heating due to interaction with RF field. Less than 1 degree is expected.
- Risk of vibration or induction of currents by the oscillating magnetic field gradients applied for the spatial encoding of the MRI signal. Due to the size of the implant this is negligible.
- Artifact in the MRI image. For ferromagnetic clips up to 4 cm, otherwise typically <1 cm.
- Risk of forces due to the Lenz effect during rapid movement of conductive implants in the static magnetic field of the MRI scanner. Due to the size of the implant this is negligible.
- Risk of Implant Disruption. We concluded that the only significant risk of disruption is in the displacement and rotation, considered under 1.
Recommendation
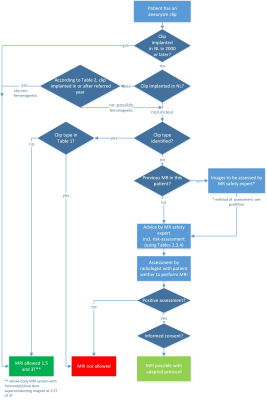
The recommendation is presented in a flowchart in Figure 1. The whole guideline can be found in the guideline database of the FMS 18.An English translation will appear early 2021. The guideline will be reviewed in five years, or earlier when necessary.
Acknowledgements
The guideline development was financed by the Quality Funds for Medical Specialists (Stichting Kwaliteitsgelden Medisch Specialisten: SKMS). We thank Diana P Gutierrez for secretary support of the guideline project, and Marco JW Götte and Evert J Vonken for participating in the working group.References
1. Mammourian A, et al. Aneurysm Clip. J Neurosurg. 2007;107:1278-9.
2. Kanal E, Barkovich AJ, Bell C, et al. Expert Panel on MR Safety. ACR guidance document on MR safe practices: 2013. J Magn Reson Imag. 2013;37:50-130.
3. Adviescommissie Richtlijnen van de Raad Kwaliteit. Medisch Specialistische Richtlijnen 2.0; 2012; http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html.
4. Brouwers M, Kho ME, Browman GP, et al. on behalf of the AGREE Next Steps Consortium: AGREE II: Advancing guideline development, reporting and evaluation in healthcare. CMAJ; 2010; www.agreetrust.org.
5. MagResource, MR:comp GmbH, Gelsenkirchen, Germany; www.MagResource.com.
6. Klucznik RP, Carrier DA, Pyka R, Haid RW. Placement of a ferromagnetic intracerebral aneurysm clip in a magnetic field with a fatal outcome. Radiology 1993;187:855-6.
7. Becker RL, et al. MR imaging in patients with intracranial aneurysm clips. Am J Neuroradiol. 1988;9:885-9.
8. Johnson GC. Need for caution during MR imaging of patients with aneurysm clips. Radiology 1993;188:287-8.
9. New, PF, et al. Potential hazards and artifacts of ferromagnetic and nonferromagnetic surgical and dental materials and devices in nuclear magnetic resonance imaging. Radiology 1983;147:139-48.
10. Shellock FG and Kanal E. Yasargil aneurysm clips: evaluation of interactions with a 1.5-T MR system. Radiology 1998;207:587-91.
11. Lauer UA, et al. Radio frequency versus susceptibility effects of small conductive implants--a systematic MRI study on aneurysm clips at 1.5 and 3 T. Magn Reson Imaging 2005;23:563-9.
12. Ooka K, et al. Motion and image artifacts of various intracranial aneurysm clips in a magnetic field. Acta Neurochir. (Wien) 1996;138(10):1241-5.
13. Watanabe A, Seguchi T, Koyama J, et al. Investigation of radiofrequency-induced temperature elevation of aneurysm clips in a 3.0-tesla magnetic resonance environment. Neurosurgery 2007;61:1062-1066.
14. Kanal, E, et al. Aneurysm clip testing for ferromagnetic properties: clip variability issues. Radiology 1996;200:576-8.
15. Shellock FG, et al. Aneurysm clips: evaluation of MR imaging artifacts at 1.5 T. Radiology 1998;209:563-6.
16. Klucznik RP, Carrier DA, Pyka R, Haid RW. Placement of a ferromagnetic intracerebral aneurysm clip in a magnetic field with a fatal outcome. Radiology 1993;187:855-6.
17. Communication MRI Safety Group ISMRM, Sept 2016.
18. Guideline database, Federation Medical Specialists, the Netherlands; https://richtlijnendatabase.nl/richtlijn/gebruik_mri_bij_patienten_met_implantaten/startpagina_-_mri_bij_patienten_met_implantaten.html
Figures