2282
Importance of Pacemaker Lead Preconditioning for MR Safety In-Vitro Studies
David Prutchi1, Jason Meyers1, and Ramez Shehada2
1Impulse Dynamics (USA) Inc., Marlton, NJ, United States, 2Medical Technology Laboratories, La Mirada, CA, United States
1Impulse Dynamics (USA) Inc., Marlton, NJ, United States, 2Medical Technology Laboratories, La Mirada, CA, United States
Synopsis
This study investigates the change in the RF filtering characteristics of the leads of active implantable medical devices (AIMDs) as body fluids seep into the leads during the initial post-implant period. Our findings indicate that the RF characteristics change dramatically with fluid absorption, making it necessary to precondition the leads by soaking in isotonic saline solution to simulate the in-vivo scenario when conducting in-vitro MR safety testing. Furthermore, leads designed with RF-attenuating lumped inductances must consider the effect of fluid absorption on changing the peak RF attenuation frequency.
Introduction
The RF currents induced into the lead of an active implantable medical device (AIMD) are transmitted through the lead body to reach its electrodes and cause heating at the tissue interface. Accordingly, the heating of the tissue will depend on the transmission line characteristics of the lead body and its ability of conducting the RF currents to the electrodes. The characteristic impedance refers to the equivalent resistance of a transmission line, owing to distributed capacitance and inductance as the voltage and current waves propagate along its length. Following implantation, conductive body fluids seep into the body of the lead to replace the air between the lead conductors thereby changing the characteristic impedance (Zo) of the lead at the RF frequencies of the MRI environment. In this study we investigated the post implantation change in Zo as the body fluids replace the air in-between the lead conductors.Methods and Results
To demonstrate the effect of liquid medium absorption by pacemaker leads on their transmission-line characteristics, we measured the open- and short-terminated impedances of a number of current MR-conditional lead samples before and after soaking them for 10 days in isotonic saline solution (0.9% NaCl) at 37°C. Results using an Anritsu MS46522A, 8.5GHz bandwidth vector network analyzer (VNA) are shown in Table 1. The characteristic impedance Z0 of the lead can be calculated from measurements of the open (unterminated) lead impedance Zopen and the short-circuit-terminated impedance Zshort:$$Z_0 = \sqrt{Z_{open}*Z_{short}}$$
We also measured the complex impedance of the leads in the ASTM F2182-191 thorax phantom filled with gel slurry of 1.32 g/l NaCl and 10g/l partial sodium salt of polyacrylic acid (PAA) with a measured conductivity of 4 mS/cm at 30°C. Figure 1 presents the magnitude and phase of the Biotronik and Abbott leads, while Figure 2 presents those for the Boston Scientific leads.
Discussion
In all pacemaker leads studied, the characteristic impedance at 63.87 MHz dropped after soaking, with an average of change of 32.99%. Figure 3 shows the Smith chart for the Biotronik Solia-S 60 lead, which presents a typical behavior. The impedance changes approximately along a constant X circle (in Z=R+jX), suggesting a change in the resistive properties of the lead as it is soaked.Another interesting observation is that in the measurements and the graphs of Figure 2, The Boston Scientific Ingevity+ leads clearly present as outliers. This is because the outer coil in these leads is made of ETFE-insulated filars2, thus increasing the outer coil’s inductance. As the leads soak, their impedance decreases together with an increase in the frequency at which the impedance peaks. Presumably, the lead is designed to stabilize such that the peak would occur in the proximity of the 63.87 MHz 1.5 T MRI frequency. However, the conditions necessary for this seem prone to be sensitive on lead aging.
Conclusion
The results above indicate that it is critical to precondition the leads by soaking them in an isotonic saline solution to simulate the in-vivo scenario prior to conducting MR safety testing in-vitro. This step is necessary to avoid misleading safety results as the diffusing body fluids change the peak impedance of the lead. Furthermore, the design of MR Conditional leads with RF-filtering lumped inductances must consider the effect of post-implantation fluid diffusion in shifting the peak RF attenuation frequency of the lead.Acknowledgements
This work was supported by Impulse Dynamics (USA) Inc.References
- ASTM F2182-19, Standard Test Method for Measurement of Radio Frequency Induced Heating On or Near Passive Implants During Magnetic Resonance Imaging, 2019.
- Boston Scientific, INGEVITY™+ Pacing Lead - Active Fixation Models: 7840, 7841, 7842 – Specification Sheet, CRM-699008-AA, 2020.
Figures
Table 1: Characteristic Impedance at
63.87 MHz of pacemaker leads before and after being soaked for over 10 days in
saline solution
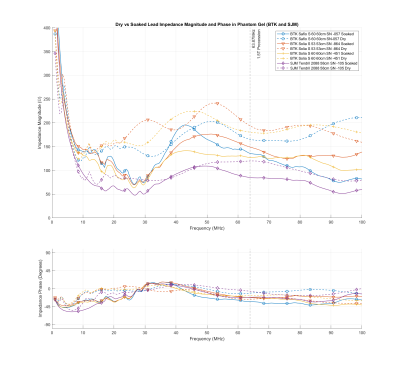
Figure 1: Magnitude and phase of
the Biotronik and Abbott leads before and after soaking for over 10 days in
isotonic saline solution
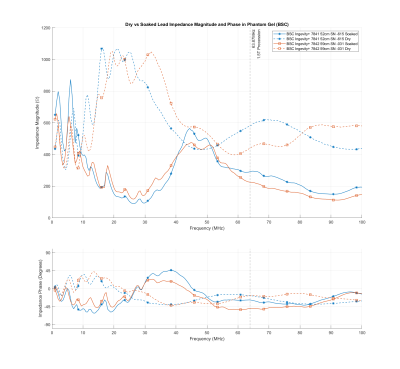
Figure 2: Magnitude and phase of
the Boston Scientific Ingevity+ leads before and after soaking for over 10 days
in isotonic saline solution.
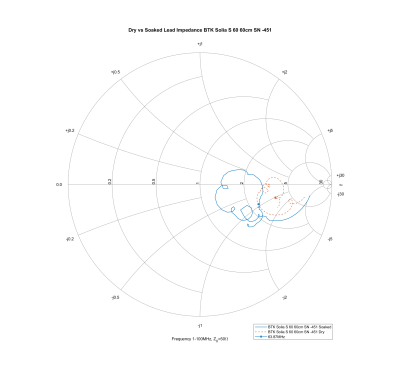
Figure 3: Smith chart of the Solia S-60 lead before and
after soaking for over 10 days in
isotonic saline solution.