2267
Design of a neonatal head and cardiac imaging system and parallel-transmit volume/receive phased array for 7T MRI during early infancy1Biomedical Engineering, Kings College London, London, United Kingdom, 2Centre for the developing brain, Kings College London, London, United Kingdom, 3Bioengineering, Imperial College London, London, United Kingdom, 4Paediatric neurosciences, Evelina London children's hospital, Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom, 5MRC Centre for Neurodevelopmental Disorders, Kings College London, London, United Kingdom
Synopsis
Magnetic resonance imaging (MRI) of the developing brain and heart could greatly benefit from the higher signal levels and improved tissue contrast at 7T. However, there are currently no dedicated RF coils and associated patient handling for young infants (<3-months). We describe design criteria and solutions to build the first mechanical frame and coil formers for imaging the brain and heart in infants up to 3-months of age in an integrated manner at 7T. The different parts incorporate important design aspects including mechanical sturdiness/safety, toxicity, sanitization and ease of use.
Introduction
Magnetic resonance imaging (MRI) of the developing brain and heart could greatly benefit from the higher signal levels and improved tissue contrast at 1-4. However, while adult safe positioning is ensured by the mechanical structure of the MR scanner, and first studies of neonatal brain have been published5, there are currently no dedicated RF coils and associated patient handling for young infants (<3-months). In this study, we describe design criteria and solutions to build the first mechanical frame and coil formers for imaging the brain and heart in infants up to 3-months of age in an integrated manner at 7T.Methods and Results
The main design criteria for the setup were established after consultation with a multidisciplinary team experienced in the field of neonatal MRI.1.Infants up to 3-months of age must fit – with size ranges to be determined from biometric data.
2.The infant should be able to be prepared away from the 7T system with all receiver coils (Rx-arrays) in-place, and then transported to the magnet with minimal further disturbance.
3.The infant needs to be accessible and covering structures should be fast to remove in emergencies.
4.The design of the patient handling and the Rx-arrays geometry must be such that Rx-arrays can be positioned in close proximity to the organs-of-interest, despite a wide range of infants body habitus.
5.The structures should allow for a 64-channel Rx-array to fit with the associated circuitry.
6.The full setup must fit into a dedicated transmit coil array since there are no integrated body coils at 7T.
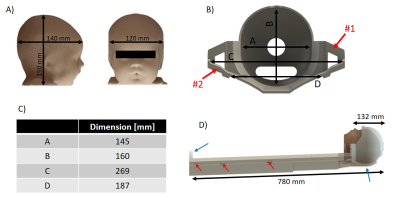
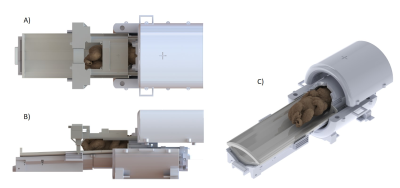
The biometric data for infants sizes6-9 determined the bed design (length=780mm,weight=2.5kg) to place the infant with its head ready to go inside a head Rx-array (Fig.1B). Helmet’s size (Fig.2B-C) included additional space to allow placement of ear protection and immobilization devices for the head. The apertures at the apex of the helmet were added for optimal head positioning9. The U-shape of the bed surface (Fig.2B) prevents lateral movement of the infant, while ensuring close proximity between Rx-arrays and the organs-of-interest. The general thickness of the bed (10mm) and the side-ears (Fig.2B,arrows #1) ensure high robustness against flexion, while regions near the brain and posterior Rx-array (pRx) kept a thickness of 4-5mm. Such precautions are essential as the bed structure is carried with one hand on a dedicated handle and the other under the helmet (Fig.2D,blue-arrows). Therefore, the centre of gravity of the infant is only supported by the bed structure. Small Teflon pads (20x15mm2) ensure the sliding of the neonatal bed over the pRx-frame and alignment (Fig.2D,red-arrows).
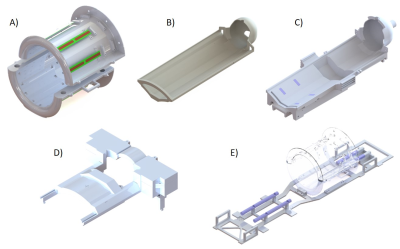
The pRx-frame supports the bed. The large hollow area (Fig.1C) enables safe and quick positioning of the bed. Supported by the Teflon rails of the main support structure (Fig.1E,blue-pieces), it can slides with minimal friction inside the Tx-array holder between the brain/heart-isocentred positions without direct interaction with the infant placed on the bed. The final position is held using star knobs easy to release.
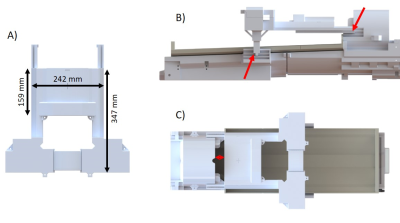
The anterior Rx-array (aRx) frame is independent of the rest of the structure, and can easily be placed or quickly removed. The designed dimensions provide a full coverage of the infant chest, while keeping an open-design to reduce parental anxiety and give radiographers a view of the infant once positioned. The aRx-frame can slide over 67mm along the longitudinal axis to enable flexible alignment with the heart position of different infants. The min/max distance between the helmet and aRx-frame was 40/107mm so the mouth and nose regions are not obstructed (Fig.3C,red-arrow). It can also move along the vertical axis so that the distance between the aRx-frame and the bed can vary from 130mm to 160mm. The helmet cover shown in Fig.3B-C supports the frame together with the pRx-frame (Fig.3B,red-arrows).
All the setup is fitting inside the Tx-array holder for the volume dipole-antenna array (Fig. 1A)10, opened at both-ends for free movement of the neonatal frame. The bed dimensions and available space inside the MR scanner bore (600mm-diameter) determined the inner/outer diameters (286/406mm) of the coil holder that was separated in two halves to provide an open view when placing infants.
The main support structure (Fig.1E) sits on the surface of the standard MR scanner bed. Through the sliding rails, it transfers the pressure of neonatal structures to the scanner ground for vertical stability. Four handles are placed along the part for transport.
All pieces were 3D-printed in polycarbonate (Deed3D,China), which guarantee MR transparency and mechanical robustness. When feasible, they were printed as one-piece to prevent the use of glue that could alter sturdiness (max.size:914x610x914mm). All geometrical features (angles,chamfers,screw-clearances,filets) were designed so that the outer surfaces could be easily sanitized. Any exposed surfaces were also painted with a lacquer meeting the international standards in terms of lack of toxicity.
Discussion and Conclusion
In this work, we describe the first mechanical frame for infants for brain and cardiac imaging at 7T. The different parts incorporate important design aspects including mechanical sturdiness/safety, toxicity, sanitization and ease of use. The Rx-array holders were designed to maximize signal in the brain and cardiac regions of infants up to 3-months of age and to fit a 64-channel Rx-array.Acknowledgements
This work was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
[1] J. T. Vaughan et
al., ‘7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in
head images’, Magnetic Resonance in Medicine, vol. 46, no. 1, pp. 24–30,
Jul. 2001, doi: 10.1002/mrm.1156.
[2] M.
I. Karamat, S. Darvish-Molla, and A. Santos-Diaz, ‘Opportunities and Challenges
of 7 Tesla Magnetic Resonance Imaging: A Review’, Crit Rev Biomed Eng,
vol. 44, no. 1–2, pp. 73–89, 2016, doi: 10.1615/CritRevBiomedEng.2016016365.
[3] A.
G. van der Kolk, J. Hendrikse, J. J. M. Zwanenburg, F. Visser, and P. R.
Luijten, ‘Clinical applications of 7 T MRI in the brain’, European Journal
of Radiology, vol. 82, no. 5, pp. 708–718, May 2013, doi:
10.1016/j.ejrad.2011.07.007.
[4] S.
Trattnig et al., ‘Clinical applications at ultrahigh field (7 T). Where
does it make the difference?’, NMR in Biomedicine, vol. 29, no. 9, pp.
1316–1334, Sep. 2016, doi: 10.1002/nbm.3272.
[5] K.
V. Annink et al., ‘Introduction of Ultra-High-Field MR Imaging in
Infants: Preparations and Feasibility’, American Journal of Neuroradiology,
Jul. 2020, doi: 10.3174/ajnr.A6702.
[6] C.
E. Sanchez, J. E. Richards, and C. R. Almli, ‘Neurodevelopmental MRI brain
templates for children from 2 weeks to 4 years of age’, Dev Psychobiol,
vol. 54, no. 1, pp. 77–91, Jan. 2012, doi: 10.1002/dev.20579.
[7] C.
R. Almli, M. J. Rivkin, and R. C. McKinstry, ‘The NIH MRI study of normal brain
development (Objective-2): Newborns, infants, toddlers, and preschoolers’, NeuroImage,
vol. 35, no. 1, pp. 308–325, Mar. 2007, doi: 10.1016/j.neuroimage.2006.08.058.
[8] J.
E. Richards, C. Sanchez, M. Phillips-Meek, and W. Xie, ‘A database of
age-appropriate average MRI templates’, Neuroimage, vol. 124, no. Pt B,
pp. 1254–1259, Jan. 2016, doi: 10.1016/j.neuroimage.2015.04.055.
[9] E.
J. Hughes et al., ‘A dedicated neonatal brain imaging system’, Magnetic
Resonance in Medicine, vol. 78, no. 2, pp. 794–804, 2017, doi:
10.1002/mrm.26462.
[10] J.
Clément et al., ‘8-channel dipole array for 7 Tesla neonatal brain and
cardiac MRI’, presented at the 28th Annual Meeting of ISMRM, 4618, Online.
[11] S.
J. Malik, J. W. Hand, R. Satnarine, and J. V. Hajnal, ‘SAR and temperature in
neonate models resulting from exposure to 7T head coil’, presented at the 28th
Annual Meeting of ISMRM, 4616, 2020.
Figures