2263
A Densely Connected Neural Network with Frequency Balancing Loss for Adipose Tissue Segmentation in Children using Free-Breathing Abdominal MRI1Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Cognitive Science, University of California, Los Angeles, Los Angeles, CA, United States, 4Pediatrics, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Obese children have larger amounts of subcutaneous and visceral adipose tissue (SAT, VAT) and are at high risk for cardiometabolic disease. The reference standard to analyze SAT/VAT uses breath-held (BH) abdominal MRI for manual annotation of SAT/VAT. In children, the BH requirement and spatially varying VAT distribution are major challenges for body composition analysis. This work proposed a densely connected neural network with a class frequency balancing, boundary emphasizing loss to segment SAT/VAT using free breathing abdominal MRI in children.
Introduction
MRI can quantify the subcutaneous and visceral adipose tissue (SAT, VAT), which are associated with high risk for cardiometabolic disease1-4. However, conventional abdominal MRI requires breath-holding (BH) to overcome motion sensitivity. BH is more challenging in children than in adults5. 3-D stack-of-radial free-breathing (FB) MRI methods can accurately quantify adipose tissue (AT) in children6, but the analysis required labor-intensive manual annotation. Researchers proposed deep learning (DL) methods to segment AT in adults, based on data from large-scale studies7-10. The lack of datasets with reliable pediatric abdominal MRI has contributed to the absence of studies investigating DL-based AT quantification in children. Additionally, VAT distribution may have more spatial variation in children than in adults, resulting in greater class imbalance between SAT and VAT11. Therefore, we proposed a densely connected neural network with a class frequency balancing, boundary emphasizing loss to segment SAT/VAT using FB abdominal MRI in children.Methods
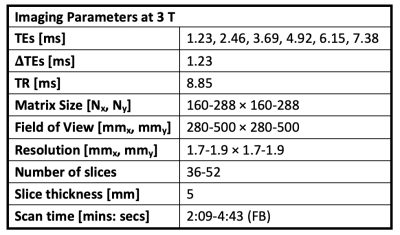
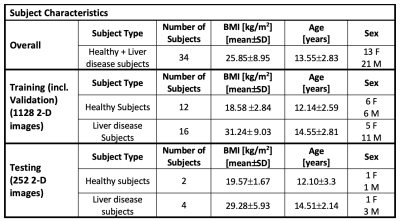
Datasets: In this IRB-approved prospective study, 34 children (healthy, or with chronic liver disease) were scanned with FB multi-echo radial MRI (Table 1) to obtain 3-D images and proton-density fat fraction (PDFF) maps.SAT and VAT were manually annotated by trained researchers and confirmed by an expert pediatric radiologist. The dataset was split into separate training (n=28/34, 85%) and testing (n=6/34, 15%) sets, with similar body mass index (BMI) (Table 2). Five subjects were randomly selected from the training set and set aside as a validation set for hyperparameter tuning. Once the hyperparameters were selected, the training and validation sets were combined.
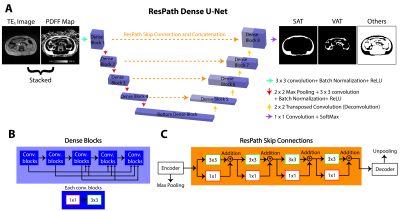
Networks: We first considered U-Net, a common baseline method for AT segmentation in adults8,12. U-Net13 was trained with weighted Dice loss (WDL) with class weights, $$$\omega_l$$$ ($$$l$$$: classes, $$$r_{ln}$$$ and $$$p_{ln}$$$: reference and output segmented pixel $$$n$$$, respectively)14. $$WDL = 1-2\frac{\sum_{l=1}^{3}\omega_l\sum_{n}r_{ln}p_{ln}}{\sum_{l=1}^{3}\omega_l\sum_{n}r_{ln}+p_{ln}}$$ $$\omega_l = \frac{1}{({\sum_{n=1}^N r_{ln}})^2}$$ Our proposed network, ResPath-DenseU-Net (Figure 1a), combines dense connections (Figure 1b) and ResPath skip connections (Figure 1c). DenseU-Net improves the generalizability of the features15,16. ResPath mitigates semantic gaps when combining the encoder and decoder features17. Due to imbalanced nature of SAT/VAT pixels and more spatially varying VAT structures in children, we trained our network with a novel frequency balancing Dice loss (FBDL) function. We used logistic weights18 for $$$\omega_l$$$ in WDL, where the first term considers the class imbalance and second term emphasizes the boundaries ($$$I$$$: indicator function, $$$f$$$: class frequencies, $$$\triangledown$$$: gradient). $$$\omega_0 = 2\frac {median(f)}{f_{min}}$$$ gives higher priority to boundaries.
$$\omega_l = \sum_{n=1}^N I(p_{ln}==l)\frac{median(f)}{f_l}+\omega_0 I(|\triangledown p_{ln}|>0)$$
The inputs to the network were the first echo time images (TE1) and PDFF maps, and the outputs were segmentation maps of SAT, VAT, and others.
Evaluations: (1) Segmentation accuracy was assessed with respect to manual annotations. 2-D Dice scores per slice (2-D Dice-SAT, Dice-VAT) were reported as mean$$$\pm$$$ standard deviation (SD) across testing subjects. 3-D Dice scores (3-D Dice-SAT, Dice-VAT) were calculated per subject. (2) AT quantification accuracy was assessed in terms of per subject mean PDFF (SAT/VAT-PDFF) and volume (SAT/VAT-Volume).
Results
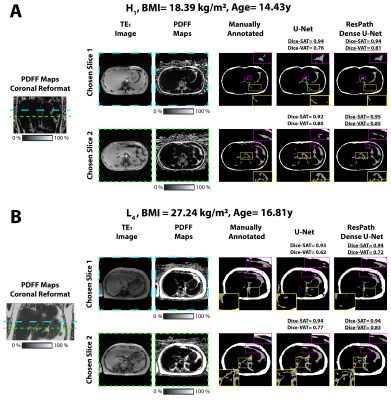
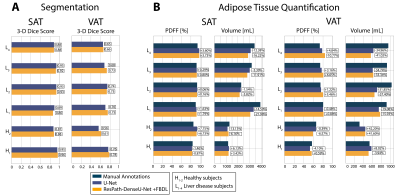
The total training time and inference time were [54 minutes, 41ms/slice] for U-Net and and [69 minutes, 67ms/slice] for ResPath-DenseU-Net. Figure 2 shows representative images from two subjects. 2-D Dice-SAT scores for the chosen slices were similar for both networks, while ResPath-DenseU-Net produced higher 2-D Dice-VAT scores. 3-D Dice-SAT scores were comparable for both networks (Figure 3a). 3-D Dice-VAT scores were mostly higher for ResPath-DenseU-Net. Additionally, ResPath-DenseU-Net produced comparable per slice 2-D Dice-SAT scores (U-Net: 0.91$$$\pm$$$0.06, ResPath-DenseU-Net: 0.91$$$\pm$$$0.09) and higher scores for VAT (U-Net: 0.58$$$\pm$$$0.26, ResPath-DenseU-Net: 0.62$$$\pm$$$0.31). Both networks achieved comparable per subject mean PDFF and volume measurements for SAT (Figure 3b). U-Net yielded VAT-Volume slightly closer to manual annotations than ResPath-DenseU-Net.Discussion
There are no published DL-based AT quantification studies in children. DL-based approaches can achieve rapid inference time on the order of ms/slice and can potentially make body composition analysis in children a practical tool for research. To develop new DL-based segmentation methods in children, reliable techniques, such as FB abdominal MRI, are essential for collecting high-quality data. For this reason, we used FB abdominal MRI as inputs to our networks. We investigated the baseline performance of U-Net for AT segmentation in children and then proposed a new network with FBDL to overcome the challenges associated with VAT distribution in children. With the novel FBDL and network improvements, ResPath-DenseU-Net improved VAT segmentation performance. The PDFF and volume measurements for both networks were close to manual annotations with the exception of underestimated VAT volumes in children with liver disease. We suspect the underestimation could be a result of small sample size and wide range of BMI for the children with liver disease.As we continue to collect more data, we also plan to investigate transfer learning based on pre-training with adult data. Our networks did not consider 3-D context of the tissue structures. Future work incorporating 3-D DenseU-Net15,19 based networks could be advantageous to learn through-plane VAT associations.
Conclusion
We proposed a densely connected network with a frequency balancing loss to segment AT in healthy children and children with liver disease using FB abdominal MRI. Our proposed network could be a semi-automated tool for researchers to rapidly analyze body composition in children.Acknowledgements
This project was supported by the UCLA Radiological Sciences Exploratory Research Program, the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK124417), and the National Center for Advancing Translational Sciences (UL1TR001881).References
1. Despres JP. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Can J Cardiol 2012;28(6):642-652.
2. Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008;28(6):1039-1049.
3. Linge J, Borga M, West J, et al. Body Composition Profiling in the UK Biobank Imaging Study. Obesity (Silver Spring) 2018;26(11):1785-1795.
4. Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol 2012;85(1009):1-10.
5. Jaimes C, Gee MS. Strategies to minimize sedation in pediatric body magnetic resonance imaging. Pediatric radiology 2016;46(6):916-927.
6. Ly KV, Armstrong T, Yeh J, et al. Free-breathing Magnetic Resonance Imaging Assessment of Body Composition in Healthy and Overweight Children: An Observational Study. J Pediatr Gastroenterol Nutr 2019;68(6):782-787.
7. Shen N, Li X, Zheng S, et al. Automated and accurate quantification of subcutaneous and visceral adipose tissue from magnetic resonance imaging based on machine learning. Magn Reson Imaging 2019;64:28-36.
8. Küstner T, Hepp T, Fischer M, et al. Fully Automated and Standardized Segmentation of Adipose Tissue Compartments via Deep Learning in 3D Whole-Body MRI of Epidemiologic Cohort Studies. Radiology: Artificial Intelligence 2020;2(5):e200010.
9. Estrada S, Lu R, Conjeti S, et al. FatSegNet: A fully automated deep learning pipeline for adipose tissue segmentation on abdominal dixon MRI. Magnetic Resonance in Medicine 2020;83(4):1471-1483.
10. Sadananthan SA, Prakash B, Leow MKS, et al. Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. Journal of Magnetic Resonance Imaging 2015;41(4):924-934.
11. Kafali SG, Shih S-F, Li X, et al. Fully Convolutional Networks for Adipose Tissue Segmentation Using Free-Breathing Abdominal MRI in Healthy and Overweight Children. 28th Annual Meeting of International Society of Magnetic Resonance in Medicine. Paris, France; 2020.
12. Langner T, Hedström A, Mörwald K, et al. Fully convolutional networks for automated segmentation of abdominal adipose tissue depots in multicenter water–fat MRI. Magnetic resonance in medicine 2019;81(4):2736-2745.
13. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. International Conference on Medical image computing and computer-assisted intervention: Springer; 2015. p. 234-241.
14. Sudre CH, Li W, Vercauteren T, Ourselin S, Cardoso MJ. Generalised dice overlap as a deep learning loss function for highly unbalanced segmentations. Deep learning in medical image analysis and multimodal learning for clinical decision support: Springer; 2017. p. 240-248.
15. Zeng Y, Chen X, Zhang Y, Bai L, Han J. Dense-U-Net: densely connected convolutional network for semantic segmentation with a small number of samples. Tenth International Conference on Graphics and Image Processing (ICGIP 2018). Volume 11069: International Society for Optics and Photonics; 2019. p. 110692B.
16. Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. Proceedings of the IEEE conference on computer vision and pattern recognition; 2017. p. 4700-4708.
17. Ibtehaz N, Rahman MS. MultiResUNet : Rethinking the U-Net architecture for multimodal biomedical image segmentation. Neural Networks 2020;121:74-87.
18. Roy AG, Conjeti S, Navab N, Wachinger C, Initiative AsDN. QuickNAT: A fully convolutional network for quick and accurate segmentation of neuroanatomy. NeuroImage 2019;186:713-727.
19. Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2016. Cham: Springer International Publishing; 2016. p. 424-432.
Figures