2258
Alterations in vascular injury, rsfMRI signal variability and verbal memory in young patients treated with radiation therapy for a brain tumor.
Melanie Morrison1, Sivakami Avadappian1, Angela Jakary1, Erin Felton2, Schuyler Stoller3, Sabine Mueller3, and Janine Lupo1
1Radiology, UCSF, San Francisco, CA, United States, 2UCSF, San Francisco, CA, United States, 3Neurology, UCSF, San Francisco, CA, United States
1Radiology, UCSF, San Francisco, CA, United States, 2UCSF, San Francisco, CA, United States, 3Neurology, UCSF, San Francisco, CA, United States
Synopsis
This is a multimodal 7T MRI study evaluating imaging biomarkers of cognition in young brain tumor patients.
Synopsis
This is a 7T multimodal imaging study evaluating imaging biomarkers of cognition in young brain tumor patients.Introduction
Introduction: Despite playing a critical role in the management of brain tumors, radiation therapy (RT) has long been associated with side effects including cognitive impairments and vascular brain injury.1,2 Previously, our group demonstrated network-level associations between temporal resting-state functional MRI (rsfMRI) metrics, cognitive performance scores, and clinical risk factors for RT-induced side effects including the irradiated brain volume, time since treatment and age during treatment.3 Such findings support the potential utility of rsfMRI for elucidation of functional substrates of RT-induced cognitive impairments. RT-induced vascular injury in form of arterial thinning and cerebral microhemorrhage detected on MRI have also been linked to cognitive status in the years following RT.4-6However, there has yet been a joint evaluation of these vascular and functional imaging biomarkers of cognitive status to determine their relative or combined clinical value. This work therefore aims to use multiparametric modeling to relate MR-derived measures of cerebroarterial density, cerebral microhemorrhage, and rsfMRI connectivity with cognitive test scores, within a young cohort of patients treated with RT for a brain tumor.Methods
Methods: We acquired rsfMRI and simultaneous MR angiography (MRA) and venography data7 (see Figure 1a for MRI parameters) at 7T in nineteen young brain tumor patients (Figure 1b; median age 18; range 12-25) who underwent cognitive assessment following imaging. Eight patients were treated with whole-brain RT, 7 patients with focal RT, and 4 were non-irradiated control brain tumor patients. The cognitive assessment consisted of a battery of computerized cognitive tests (Cogstate, Inc.; Newhaven, CT) chosen specifically to elucidate impairment in specific cognitive domains. Raw scores were converted to z-scores using mean performance scores of healthy control subjects from the Cogstate database. For this work we focused on a single verbal memory task (involving verbal recall of shopping list items Figure 1c), as we previous found this task to be most effective for distinguishing between patients according to risk factors for treatment induced side effects.4We applied a novel N4-correction to T1-weighted to improve signal inhomogeneity and tissue segmentation during rsfMRI preprocessing.7 All fMRI preprocessing was performed in the CONN toolbox8 followed by group independent component analysis with 40 components in order to extract spatially and temporally distinct large-scale brain networks (Figure 2). Here we chose to target the fronto-parietal network (FPN), default mode network (DMN), salience network (SN), and dorsal attention network (DAN) (Figure 2a) given prior evidence of their involvement in verbal and working-memory performance.9 Within each network mask, local rsfMRI signal variability – thought to represent the flexibility and adaptability of a neural system10 – was computed as the standard deviation of the signal (Figure 2b). Whole-brain cerebroarterial density was calculated from the MRA data using a novel Frangi vesselness filtering tool for vessel quantification and normalized by the total head volume (Figure 2c).12 A novel in-house tool for semi-automated microhemorrhage quantification was used to detect total cerebral microbleed burden from susceptibility-weighted imaging (Figure 2d).12,13 Multiple regression analyses were used to contrast the relationship between individual imaging metrics and verbal memory performance, as well as the interaction of imaging metrics in order to evaluate their combined effect.Results
Results: We observed group differences across the different imaging parameters with respect to the RT strategy (no RT vs. focal vs. whole), as well as the RT dose (~23 Gy vs 36 Gy) when contrasting only those patients treated with whole-brain RT (Figure 4a). Notably, cerebroarterial volume, vvol, could best distinguish the different patient groups, with those patients who received more exposure to RT exhibiting reduced vvol. A significant independent correlation between fMRI signal variability within the DAN and vvol was also observed (Figure 4b). No independent correlations were observed for cerebral microbleed burden, likely due to a limited sample size and follow-up time. Our regression analysis revealed significant relationships between vvol, DMN signal variability and verbal memory performance that were most significant (p=0.0042) when modelling vvol and DMN variability together as a joint interaction term (Figure 4).Conclusions
Conclusions: There is a strong need for reliable and reproducible biomarkers of cognition to manage young brain tumor patients in the years after RT. Results from our 7T imaging study suggest that both vascular and functional MRI metrics could be used together to assess critical cognitive domains that are often impaired as a result of treatment. Continuing to build on this, ongoing work will integrate measures of white matter structural integrity from diffusion tensor imaging, in order to develop a robust multiparametric biomarker of cognition that could serve as an adjunct to standard testing batteries.Acknowledgements
We would like to thank the patients and their families for participating in the study.References
- Greene-schloesser, D., & Robbins, M. E. (2012). Radiation-induced cognitive impairment- from bench to bedside. Neuro-Oncology, 37–44.
- Greene-Schloesser, D., Robbins, M. E., Peiffer, A. M., Shaw, E. G., Wheeler, K. T., & Chan, M. D. (2012). Radiation-induced brain injury: A review. Frontiers in Oncology, 2(July), 73.
- Morrison, M. A., Jakary, A., Felton, E., Stoller, S., Mueller, S., & Lupo, J. 1241 Functional connectivity is associated with radiotherapy-induced vascular injury and cognitive impairment in young brain tumor survivors. (2020). Proc. Intl. Soc. Mag. Reson. Med.
- Morrison, M. A., Mueller, S., Felton, E., Jakary, A., Stoller, S., Avadiappan, S., Yuan, J., Molinaro, A. M., Braunstein, S., Banerjee, A., Hess, C. P., & Lupo, J. M. (2021).
- Rate of radiation-induced microbleed formation on 7T MRI relates to cognitive impairment in young patients treated with radiation therapy for brain tumor. Radiother Oncol. 2021 Jan 1; 154: 145-153. Epub 2020 Sept 20.
- Roddy, E., Sear, K., Felton, E., Tamrazi, B., Gauvain, K., Torkildson, J., Buono, B. D., Samuel, D., Haas-Kogan, D. A., Chen, J., Goldsby, R. E., Banerjee, A., Lupo, J. M., Molinaro, A. M., Fullerton, H. J., & Mueller, S. (2016). Presence of cerebral microbleeds is associated with worse executive function in pediatric brain tumor survivors. Neuro-Oncology, 18(11), 1548–1558.
- Avadiappan, S., Morrison, M., Jakary, A., Felton, E., Stoller, S., Hess, C., Mueller, S., Braunstein, S., & Lupo, J.M. (2019). Medu-43. Global and regional effects of radiation therapy on cerebral microvasculature in pediatric brain tumor survivors. SNO Pediatric Neuro-Oncology Basic and Translational Research Conference; Oxford University Press; c2019.
- Leynes, A. P., Morrison, M. A., Jakary, A., Larson, P., Lupo, J. M. (2017). 5221 Robust Bias Correction and Segmentation of 7 Tesla Structural Brain Images with an iterative Bias-Corrected Fuzzy C-means and N4 Bias Correction (iBCFCM+N4). Proc. Intl. Soc. Mag. Reson. Med.
- Whitfield-Gabrieli S, Nieto-Castanon A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2(3):125-41.
- Jeong, W., Kee Chung, C., & Sic Kim, J. (2015). Episodic memory in aspects of large-scale brain networks. Front. Hum. Neurosci. 9:454.10. Nomi, J.S., Bolt, T. S., Chiemeka Ezie, C. E., Uddin, L. Q., Heller, A. S. (2017). Moment-to-moment BOLD signal variability reflects regional changes in neural flexibility across the lifespan. Journal of Neuroscience.37(22): 5539-5548.
- Avadiappan, S., Payabvash, S., Morrison, M. A., Jakary, A., Hess, C. P., & Lupo, J. M. (2020). A fully automated method for segmenting arteries and quantifying vessel radii on magnetic resonance angiography images of varying projection thickness. Front Neurosci. 2020; 14:537. eCollection 2020.
- Morrison, M. A., Payabvash, S., Chen, Y., Avadiappan, S., Shah, M., Zou, X., Hess, C. P., Lupo, J. M. (2018). A user-guided tool for semi-automated cerebral microbleed detection and volume segmentation: Evaluating vascular injury and data labelling for machine learning. Neuroimage Clin. 2018;20:498-505. eCollection 2018.
- Bian, W., Hess, C.P., Chang, S.M., Nelson, S. J., & Lupo, J. M. (2013). Computer-aided detection of radiation-induced cerebral microbleeds on susceptibility-weighted MR images. NeuroImage Clin. 2013;2:282–290.
Figures
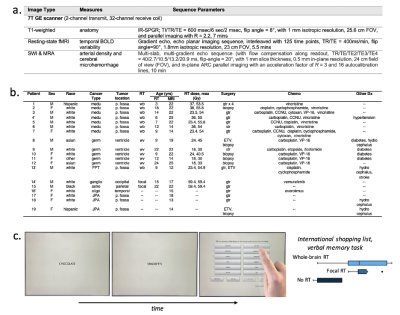
Figure 1: 7T MRI protocol (a), patient demographics (b), and an illustration of the computerized verbal memory task which best distinguished between patient RT groups in prior research (c).
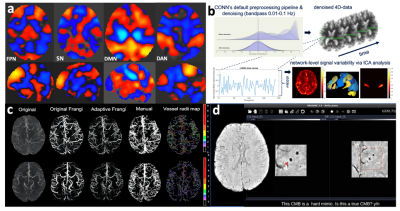
Figure 2: Group-level large-scale brain networks derived from ICA analysis (a; orange = positive correlation, blue = negative correlation; FPN = frontoparietal network, SN= salience network, DMN = default mode network, DAN = dorsal attention network) and the methods for extracting local rsfMRI variability (b), cerebroarterial density (c) and cerebral microbleed (CMB) burden (d).
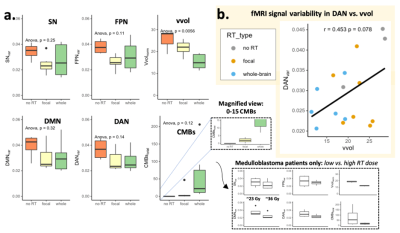
Figure 3: Group differences in imaging parameters (a) and the relationship between rsfMRI variability in the dorsal attention network (DAN) and whole brain cerebroarterial density (vvol) (b). SN = salience network, FPN = frontoparietal network, DMN = default mode network, DAN = dorsal attention network, Vvol = vessel volume (normalized), CMBs = cerebral microbleed burden. All fMRI signal variability metrics were age-normalized, while vessel volumes were normalized by the total brain volume.
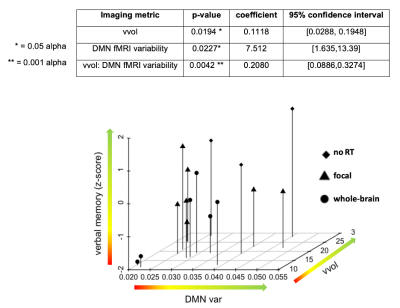
Figure 4: Relationship between imaging parameters and verbal memory performance (dependent variable). DMN var = default mode network variability, vvol= normalized vessel volume.