2257
Patterns of De Novo Myelination Identify Functionally Relevant Brain Networks1Bill & Melinda Gates Foundation, Seattle, WA, United States, 2Advanced Baby Imaging Lab, Rhode Island Hospital, Providence, RI, United States, 3Pediatrics, Rhode Island Hospital, Providence, RI, United States
Synopsis
Infancy and childhood are important developmental periods punctuated by rapid brain development and cognitive growth, however, direct and longitudinal analysis of the relationships between maturing brain structure and evolving cognitive skill in human infants is sparse. Here we used longitudinal and myelin-sensitive MRI measures from a large cohort of neurotypical children to examine patterns of myelination throughout the brain and link these patterns to concurrently evolving motor, visual, and language skills. Results revealed a core system of central brain regions that contributed broadly across cognitive domains as well as domain-specific regions that align with known functional specialization.
Introduction
The maturation of the brain’s myeloarchitecture is an important neurodevelopmental process that underpins efficient brain messaging and communication and, accordingly, is believed to contribute to the development and refinement of cognitive skills and abilities. While conceptually supported by the concurrent timelines of histological assays of myelination and cognitive development from behavioral studies, in vivo demonstration of myelin-cognitive associations across infancy and childhood is limited. From histology, myelination proceeds in a caudal-cranial and posterior-to-anterior arc driven by the increasingly coherent synaptic activity associated with emerging cognitive processing and functioning. Patterns of myelination, therefore, may inform on the rate and sequence of cognitive development, as well as anatomical-functional specialization.Methods
A total of 1290 longitudinal neuroimaging datasets for this study were obtained from 587 (259 females) healthy and neurotypically-developing children, with data spanning 45 days through 13.4 years of age on a Siemens 3T Trio with a 12-channel head RF array. Children under 4 years of age were imaged during non-sedated sleep and older children whilst watching a movie. Children were swaddled with a MedVac immobilizer and foam cushions to minimize motion, and scanned with acoustically derated sequences. mcDESPOT(1) was used to estimate myelination via the myelin water fraction (MWF)(2).Brain regions with similar myelination patterns were identified using probabilistic independent component analyses(3) to delineate statistically independent spatial regions (ICs) with shared temporal myelination trajectories. Cognitive ability and verbal and non-verbal functioning was assessed in children under 4 (N=450, 199 female) using the Mullen Scales of Early Learning (MSEL(4)).
Using the imaging and neurocognitive data, we identified the ICs significantly associated with cognitive development by fitting linear mixed-effect models for myelination
$MWFi,j = βo,j + β1,jlog(age)$
and for cognition (fine motor, FM, as example) .
$FMi,j = βo,j + β1,j(age)$
The correlation between the random estimates (β1,j) of myelin and cognitive change (slope) were then calculated for each IC and cognitive measure, and significant pairs (p<0.05FDR) identified.
Results & Discussion
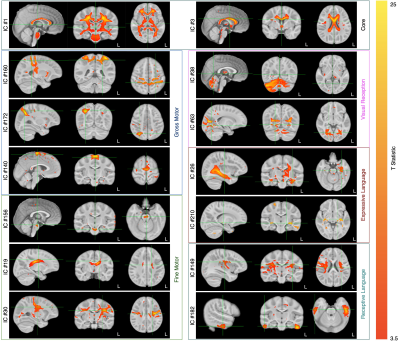
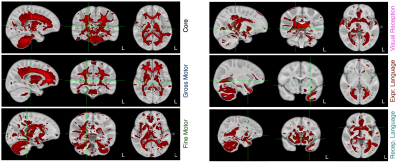
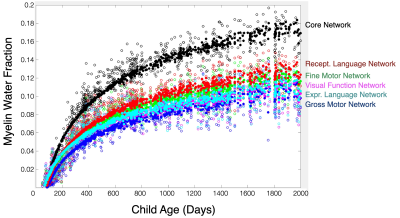
175 ICs were identified, accounting for 80% of the total dataset variance. In 14, the rate of myelination was statistically associated with the rate of gross motor development; 65 with fine motor development; 72 with visual function; and 43 and 77 with expressive and receptive language skill maturation, respectively. Across the fine motor, language, and visual domains, we found 34 shared ICs that made up a common ‘core’ network. A subset of ICs associated with each functional domain are shown in Fig. 1 with composite network maps, constructed by merging the individual IC maps associated with each functional domain are shown in Fig. 2. Mean myelin water fraction trajectories for the 6 composite networks are shown in Fig. 3. Examining the mean myelination trajectories associated with the composite networks we find the following average logarithmic rates of development: Common network: 0.055 MWF/log(days); Gross Motor: 0.034 MWF/log(days); Fine Motor: 0.037 MWF/log(days); Visual Reception: 0.031 MWF/log(days); Expressive Language: 0.033 MWF/log(days), and Receptive Language: 0.038 MWF/log(days). The core network, comprising the corpus callosum, cerebellum, brain stem and basal ganglia, and internal capsule, therefore, exhibits fast and early maturation, allowing it to play an underlying role to more specialized functional domains. Building on top of this core network, we identified more functionally specific regions. Fine motor, including somatosensory and motor cortices, the pons, cerebellum, corpus callosum, caudate nucleus, putamen, thalamus, orbital frontal cortex, and occipital and visual cortex. These regions have well known connections with each other, for example cortical-ponto-cerebellar and cerebello-thalamic-cortical pathways linking the cerebellum, pons, and thalamus to visual and motor areas(5); striatal-cortical connections including the caudate nucleus, putamen, and motor areas; and orbital frontal connections to secondary motor areas(6). While many fine motor areas overlap with gross motor, we also find specific and unique associations between gross motor and supplementary motor and premotor cortices, and dorsolateral and ventrolateral prefrontal cortices, and supramarginal gyrus. Though frontal lobe regions are amongst the last to myelinate(7), following many of the regions associated with fine motor function, this may more directly relate to these regions involvement in walking, balance, coordination, and spatial processing(8), skills that do not evolve until 9-12 months of age(9). The importance of visual processing to fine motor skill(10) likely underlies the overlap of brain regions we found contribute to both functions. The main difference being increased involvement of occipital white matter and visual cortex areas for visual processing, and anatomical specificity to lateral thalamic nuclei. We also find that myelination of temporal lobe regions (predominately left hemisphere), prefrontal cortex, superior and inferior longitudinal fasciculus, arcuate fasciculus, and striata regions is associated with expressive and receptive language skills. In this work, we used a data-driven approach to delineate and functionally ascribe brain networks from longitudinal myelin-sensitive MRI measures for the first time. Results provide new insights into the sequence, rate, and timing, of developing brain regions, the integrated nature of the brain’s white matter systems.Acknowledgements
No acknowledgement found.References
1. Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. Dec 2008;60(6):1372-87. doi:10.1002/mrm.21704
2. MacKay AL, Laule C. Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin. Brain Plast. Dec 21 2016;2(1):71-91. doi:10.3233/BPL-160033
3. Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. Feb 2004;23(2):137-52. doi:10.1109/TMI.2003.822821
4. E.M. M. Mullen Scales of Early Learning. American Guidance Services, Inc; 1995.
5. Habas C, Manto M. Probing the neuroanatomy of the cerebellum using tractography. Handb Clin Neurol. 2018;154:235-249. doi:10.1016/B978-0-444-63956-1.00014-X
6. Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. Mar 2016;18(1):7-21.
7. Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol. May 1987;46(3):283-301. doi:10.1097/00005072-198705000-00005
8. Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuroimage. Nov 1 2008;43(2):329-36. doi:10.1016/j.neuroimage.2008.07.029
9. Wilks T, Gerber RJ, Erdie-Lalena C. Developmental milestones: cognitive development. Pediatr Rev. Sep 2010;31(9):364-7. doi:10.1542/pir.31-9-364
10. Davis EE, Pitchford NJ, Limback E. The interrelation between cognitive and motor development in typically developing children aged 4-11 years is underpinned by visual processing and fine manual control. Br J Psychol. Aug 2011;102(3):569-84. doi:10.1111/j.2044-8295.2011.02018.x
11. Marques JP, Simonis FFJ, Webb AG. Low-field MRI: An MR physics perspective. J Magn Reson Imaging. Jun 2019;49(6):1528-1542. doi:10.1002/jmri.26637
Figures