2256
Breastmilk exposure is associated with improved MRI biomarkers of myelination in preterm infants1MRC Centre for Reproductive Health, University of Edinburgh, Edinburgh, United Kingdom, 2Department of Paediatric Radiology, Royal Hospital for Sick Children, Edinburgh, Edinburgh, United Kingdom, 3Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
Synopsis
Preterm birth is closely associated with altered white matter microstructure and dysconnectivity of developing neural networks with increased risk of long-term neurodevelopmental impairment. Nutritional exposures in the weeks after preterm birth affect head growth, brain macro- and micro- structure, and are associated with neurocognitive ability; the mechanisms underlying these associations are uncertain. By combining nutritional data with myelin-weighted imaging, we show that early breast milk exposure after preterm birth is associated with improved white matter myelination at term-equivalent age.
Introduction
Greater exposure to breast milk following preterm birth is associated with increased fractional anisotropy (FA) in white matter tracts at term-equivalent age [1]. Whilst FA is a sensitive marker of white matter maturation in preterm infants, it is influenced by a number of biological factors including axonal density, axonal diameter, and myelination, leaving uncertainty about which of these features explains the relationship between breast milk exposure and structural connectivity. Magnetisation transfer (MT) imaging is a myelin sensitive modality that has been used to estimate myelination in children born preterm, and is of particular interest because it may have prognostic value in this population [2]. MT ratio (MTR) is sensitive to myelin-associated macromolecules [3] but is also influenced by water content and cellular density. MT saturation (MTsat) offers a more accurate quantitative measurement of myelination by decoupling MTR from R1 (1/T1) [4]. We studied a neonatal cohort to test the hypothesis that breast milk exposure following preterm birth is associated with myelin-weighted imaging metrics (MTR and MTsat) at term-equivalent age.Methods
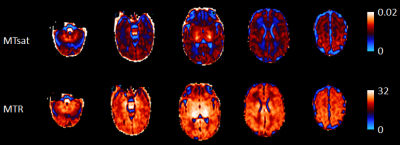
Participants: Brain MRI was performed on 62 preterm infants born before 32 weeks’ gestation at term-equivalent age as part of the Theirworld Edinburgh Birth Cohort study [5]. Ethical approval was obtained from the UK National Research Ethics Service and parents provided written informed consent. Breast milk exposure: Daily nutritional intake was collected from birth until hospital discharge using electronic patient records to identify the proportion (%) of days infants received exclusive breast milk feeds. MRI acquisition: A Siemens MAGNETOM Prisma 3 T MRI scanner (Siemens Healthcare Erlangen, Germany) with 16-channel phased-array paediatric head coil was used to acquire: A multishell axial and diffusion MRI scan (dMRI, 16×b=0s/mm2, 3×b=200s/mm2, 6×b=500s/mm2, 64×b=750s/mm2, 64×b=2500s/mm2), and sagittal 3D multiecho spoiled gradient echo scans (TE=(1.54, 4.55 and 8.56 ms), 2mm isotropic acquired resolution), magnetisation-transfer, proton density-weighted images with and without magnetisation transfer saturation (TR=75 ms, flip angle =5o) and additional T1w images (TR=15 ms, flip angle =14o)[5]. MRI processing: dMRI volumes were denoised [6], and corrected for eddy current, head movement and EPI geometric distortions [7,8]. Bias field inhomogeneity correction was then applied [9] and FA maps were obtained. MTR, MTsat and R1 maps were obtained using in-house MATLAB scripts (https://github.com/mjt320/MT-saturation), and then rigidly aligned to the mean b0 volume. The white matter core for each subject was created using the TBSS framework [10] using a neonatal specific template [11]. Once the skeleton was created, MT images were projected back onto the native space and mean MTR and MTsat values across the white matter skeleton were calculated (see Figure 1). Analysis: The relationships between the proportion of days of exclusive breast milk feeds during neonatal admission and mean white matter MTR and MTsat were assessed using Spearman’s rank correlation with adjustment for gestational age (GA) at birth and age at scan.Results
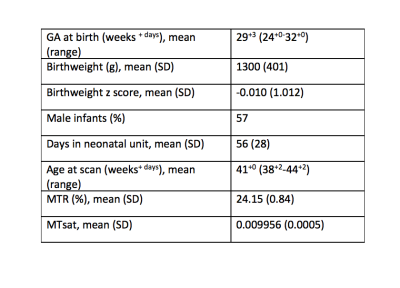
Participant demographicsCharacteristics of participants are shown in Table 1.
Early breast milk exposure is associated with myelination
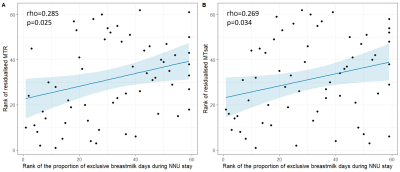
The proportion of exclusive breast milk days during neonatal admission was positively correlated with white matter MTR (rho=0.285 p=0.025) and MTsat (rho=0.269, p=0.034) after adjustment for GA at birth and age at scan (Figure 2). There was no correlation between breast milk exposure and R1.
Discussion
These MTR values are consistent with previous MT imaging studies of the neonatal brain which show increasing MTR with advancing gestational age [12]. As MT imaging is sensitive to myelin-associated macromolecules such as cholesterol and myelin basic protein but also lipids such as sphingomyelin and galactocerebrides, which are concentrated in immature oligodendrocytes, our data suggest that breast milk exposure after preterm birth is associated with oligodendrocyte maturation or myelin deposition. MTsat offers a promising tool to investigate the effect of perinatal exposures on myelination in the developing brain.Conclusion
By combining nutritional data with myelin-weighted imaging of the neonatal brain we have shown that early breast milk exposure after preterm birth is associated with improved biomarkers of white matter myelination at term-equivalent age.Acknowledgements
We are grateful to the families who consented to take part in the study. This work was supported by Theirworld (www.theirworld.org) and was undertaken in the MRC Centre for Reproductive Health, which is funded by MRC Centre Grant (MRC G1002033). KV is funded by the Wellcome Trust Translational Neuroscience PhD Programme at the University of Edinburgh (108890/Z/15/Z). MJT is supported by NHS Lothian Research and Development Office.References
1. Blesa, M., Sullivan, G., Anblagan, D., Telford, E.J., Quigley, A.J., Sparrow, S.A., Serag, A., Semple, S.I., Bastin, M.E., Boardman, J.P., 2019. Early breast milk exposure modifies brain connectivity in preterm infants. NeuroImage 184, 431-439.
2. Vandewouw, M.M., Young, J.M., Shroff, M.M., Taylor, M.J., Sled, J.G., 2019. Altered myelin maturation in four year old children born very preterm. NeuroImage. Clinical 21, 101635.
3. Mancini, M., Karakuzu, A., Cohen-Adad, J., Cercignani, M., Nichols, T.E., Stikov, N., 2020. An interactive meta-analysis of MRI biomarkers of myelin. eLife 9, e61523.
4. Helms, G., Dathe, H., Kallenberg, K. and Dechent, P. (2008), High‐resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn. Reson. Med., 60: 1396-1407
5. Boardman JP, Hall J, Thrippleton MJ, Reynolds RM, Bogaert D, Davidson DJ, et al. Impact of preterm birth on brain development and long-term outcome: protocol for a cohort study in Scotland. BMJ open. (2020) 10:e035854.
6. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. (2016) 142:394–406.
7. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. (2003) 20:870–88.
8. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. (2016) 125:1063–78.
9. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. (2010) 29:1310–20.
10. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. (2006) 31:1487–505.
11. Blesa M, Galdi P, Sullivan G, Wheater EN, Stoye DQ, Lamb GJ, Quigley AJ, Thrippleton MJ, Bastin ME and Boardman JP (2020) Peak Width of Skeletonized Water Diffusion MRI in the Neonatal Brain. Front. Neurol. 11:235.
12. Nossin-Manor, R., Card, D., Raybaud, C., Taylor, M.J., Sled, J.G., 2015. Cerebral maturation in the early preterm period-A magnetization transfer and diffusion tensor imaging study using voxel-based analysis. NeuroImage 112, 30-42.
Figures