2255
Early Sensorimotor Tract Integrity and Development of Cerebral Palsy in High Risk Infants1Perinatal Institute, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 3Division of Occupational Therapy and Physical Therapy, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 4Department of Rehabilitation, Exercise and Nutrition Sciences, University of Cincinnati College of Allied Health Sciences, Cincinnati, OH, United States, 5Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 6Imaging Research Center, Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 7Department of Radiology, University of Cincinnati College of Medicine, Cincinnati, OH, United States
Synopsis
Very preterm (VPT) infants are at high risk of motor impairments such as cerebral palsy (CP). Currently, qualitative findings from structural MRI and outcomes from clinical assessments are insufficient for early, accurate diagnosis of CP. In a multicenter cohort of 263 VPT infants, we derived macro and microstructural biomarkers of sensorimotor white matter tracts from term-equivalent age diffusion MRI. We observed consistent negative associations between these metrics – fiber density, fiber cross-section, and combined fiber density and cross-section – and early diagnosis of CP. These findings enhance our understanding of the pathophysiology of CP in VPT infants.
Introduction
Infants born very preterm (VPT, ≤ 32 weeks gestational age) are at high risk of motor impairments such as cerebral palsy (CP). Clinical diagnosis can be delayed until 2 years of age, as early neuroimaging is ‘normal’ in up to 30% of children who develop CP, and outcomes from the General Movements Assessment alone are insufficient for early, accurate prediction1. Validated biomarkers at birth are needed for earlier detection and intervention. Our objectives were to 1) use fixel-based analysis to derive micro and macrostructural measures of integrity for sensorimotor white matter tracts acquired from term-equivalent age (TEA) diffusion MRI and 2) examine the relationship between these metrics and the early diagnosis of CP.Methods
Data AcquisitionWe enrolled 263 VPT infants as part of a large prospective cohort study. Diffusion and structural MRI was acquired at TEA (3T Philips Ingenia). 68 directions of diffusion gradients were obtained: b-value of 2000 s/mm2; low b-value of 0 s/mm2; 88 ms echo time; 5073 ms repetition time; flip angle 90°; FOV 160x160 mm2, 80×78 matrix; 2-mm slice thickness; multiband factor = 2; and SENSE factor = 2.
Early Diagnosis of CP
To assess motor function, the Hammersmith Infant Neurological Examination (HINE) and Prechtl’s General Movements Assessment (GMA) were performed at 3- to 4- months corrected age by a single masked assessor. Using international consensus guidelines, we diagnosed our high-risk VPT infants with CP if they had at least two of the following: moderate to severe brain abnormality on structural MRI, abnormal HINE score, and abnormal GMA outcome2.
MRI Processing
Constrained Spherical Deconvolution (CSD) is a technique that partially overcomes limitations of the diffusion tensor model by resolving regions of crossing fibers with enhanced fidelity3. After preprocessing the b2000 diffusion weighted data, we used CSD to estimate the response function (i.e. the expected signal from a single population of white matter fibers), which was then used as the deconvolution kernel to derive the white matter fiber orientation distribution (fOD) for each subject4. The group-average fOD template was segmented to produce a group fixel template for fixel-based analyses, and probabilistic whole-brain tractography was performed (Figure 1)5,6.
Tract Segmentation
With a region of interest (ROI) based approach (Figure 2), we created seed point, waypoint, and exclusion masks in MRtrix3 that were distinct to each tract, using neuroanatomical landmarks described in our published methods1,4,7. We extracted 9 sensorimotor tracts (Figure 3) that are commonly implicated in the development of CP: the corpus callosum (CC) and the bilateral corticospinal tract (CST), posterior thalamic radiations (PTR), and superior thalamic radiations (STR, motor and sensory components).
Fixel-Based Analysis
For all tracts and subjects, we extracted fixel-based metrics, including fiber density (FD), fiber cross-section (FC), and the combined metric, fiber density and cross-section (FDC)5,6. These metrics served as our biomarkers of microscopic and macroscopic fiber integrity.
Results
225 infants had high-quality b2000 diffusion-weighted data and the follow-up at 3-4 months corrected age necessary to generate early diagnosis of CP. Their mean (SD) gestational age (GA) and postmenstrual age (PMA) at MRI scan were 29.3 (2.4) and 42.7 (1.3) weeks, respectively. 16 (7.1%) of these VPT infants were diagnosed with CP based on our a priori definition.We found widespread significant differences in fixel-based metrics between groups (Table 1). In logistic regression analysis, FD, FC, and FDC for all 9 sensorimotor tracts were significantly negatively associated with early diagnosis of CP (Table 2), with and without adjustment for significant confounders (PMA, GA, severe bronchopulmonary dysplasia, postnatal sepsis, and postnatal corticosteroids).
Discussion
We have demonstrated that CSD-derived, fixel-based measures of key sensorimotor tracts are independently associated with early diagnosis of CP in VPT infants at TEA. This is a novel finding, as no prior study has reported such robust, consistent associations between measures of micro and macroscopic fiber integrity at TEA and early motor outcomes.Using the novel fixel-based framework, we obtained a comprehensive understanding of how pathophysiological changes in fiber morphometry influence aberrant motor development in VPT infants. In our cohort, we examined relative FD and FC values as measures of axonal integrity, with higher FD indicating larger intra-axonal area per voxel or improved fiber myelination and lower FC indicating white matter atrophy6. The results allow us to assert that the pathophysiology of CP in VPT infants involves widespread decreased axonal integrity of sensorimotor tracts via diminished within-voxel fiber density and tract cross-section.
Previous diffusion tensor studies have implicated injury or immaturity to various white matter tracts in preterm infants and children with CP. However, few have reported on the relationship between tract metrics and early motor outcomes. Our results support the importance of all 9 tracts in early motor development. Furthermore, the consistent associations attest to the high statistical power of our study and superiority of CSD as a method for investigating white matter tract integrity.
Conclusion
Fixel-based biomarkers of sensorimotor tracts enhance our understanding of the macroscopic and microscopic white matter changes that antecede and potentially contribute to the development of CP in VPT infants. These biomarkers may facilitate more accurate early detection and longitudinal monitoring of treatment effects to prevent or reduce the risk of motor impairment in this vulnerable population.Acknowledgements
We sincerely thank the parents of infants that participated in our study and the Cincinnati Infant Neurodevelopment Early Prediction Study (CINEPS) Investigators: Principal Investigator: Nehal A. Parikh, DO, MS. Collaborators (in alphabetical order): Mekibib Altaye, PhD, Anita Arnsperger, RRT, Traci Beiersdorfer, RN BSN, Kaley Bridgewater, RT(MR) CNMT, Tanya Cahill, MD, Kim Cecil, PhD, Kent Dietrich, RT, Christen Distler, BSN RNC-NIC, Juanita Dudley, RN BSN, Brianne Georg, BS, Cathy Grisby, RN BSN CCRC, Lacey Haas, RT(MR) CNMT, Karen Harpster, PhD, OT/RL, Lili He, PhD, Scott K. Holland, PhD, V.S. Priyanka Illapani, MS, Kristin Kirker, CRC, Julia E. Kline, PhD, Beth M. Kline-Fath, MD, Hailong Li, PhD, Matt Lanier, RT(MR) RT(R), Stephanie L. Merhar, MD MS, Greg Muthig, BS, Brenda B. Poindexter, MD MS, David Russell, JD, Kari Tepe, BSN RNC-NIC, Leanne Tamm, PhD, Julia Thompson, RN BSN, Jean A. Tkach, PhD, Hui Wang, PhD, Jinghua Wang, PhD, Brynne Williams, RT(MR) CNMT, Kelsey Wineland, RT(MR) CNMT, Sandra Wuertz, RN BSN CCRP, Donna Wuest, AS, Weihong Yuan, PhD.References
1. Parikh NA, Hershey A, Altaye M. Early Detection of Cerebral Palsy Using Sensorimotor Tract Biomarkers in Very Preterm Infants. Pediatric Neurology 2019; 98: 53–60.
2. Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatrics 2017; 171(9): 897–907.
3. Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage 2004; 23(3): 1176–1185.
4. Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 2019; 202: 116137.
5. Raffelt D, Tournier JD, Rose S, Ridgway GR, Henderson R, Crozier S, et al. Apparent Fibre Density: A novel measure for the analysis of diffusion-weighted magnetic resonance images. NeuroImage 2012; 59(4): 3976–3994.
6. Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, et al. Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage 2017; 144: 58–73.
7. Kaur S, Powell S, He L, Pierson CR, Parikh NA. Reliability and repeatability of quantitative tractography methods for mapping structural white matter connectivity in preterm and term infants at term-equivalent age. PLoS ONE 2014; 9(1): e85807.
Figures
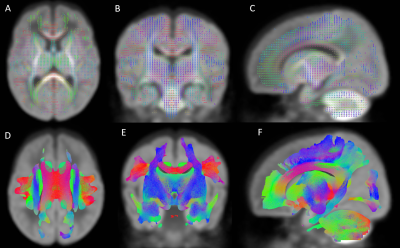
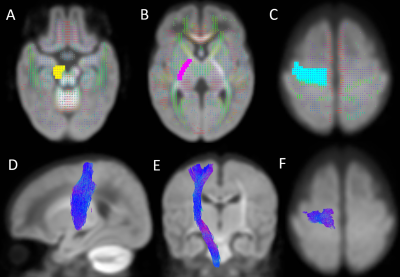
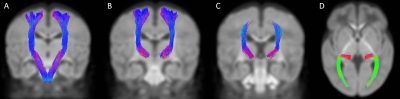
Figure 3. Bilaterally segmented sensorimotor tracts. (A) corticospinal tract in coronal view; (B) superior thalamic radiations (motor) in coronal view; (C) superior thalamic radiations (sensory) in coronal view; (D) posterior thalamic radiations in axial view
*All fibers of the STRS are not visible in coronal view, as the tract continues from the thalamus to the post-central gyrus
Table 1. Sensorimotor Tract Fiber Density (FD), Fiber Cross-Section (FC), and Fiber Density and Cross-Section (FDC) Differences Between Very Preterm Infants With and Without Early Diagnosis of Cerebral Palsy (CP).
*All values for fiber density (FD), fiber-bundle cross section (FC), and fiber density and cross-section (FDC) are corrected for postmenstrual age at MRI scan.
Table 2. Logistic Regression Analysis between Sensorimotor Tract Fiber Density and Cross-Section (FDC) and Cerebral Palsy, With and Without Adjustment for Clinical Covariates.
Abbreviations: corticospinal tract (Right = RCST, Left = LCST); superior thalamic radiations motor (Right = RSTRM, Left = LSTRM); superior thalamic radiations sensory (Right = RSTRS, Left = LSTRS); posterior thalamic radiations (Right = RPTR, Left = LPTR); corpus callosum = CC; fixel-based = FB; postmenstrual age = PMA