2252
Increased glutamate + glutamine correlates with altered tactile perception and sensory responsivity in children with autism spectrum disorder1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Forensic and Neurodevelopmental Sciences, Sackler Institute for Translational Neurodevelopment, Institute of Psychiatry, Psychology, and Neuroscience, King's College London, London, United Kingdom, 4Center for Neurodevelopmental and Imaging Research, Kennedy Krieger Institute, Baltimore, MD, United States, 5Department of Psychiatry, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 6Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Abnormal perception of sensory input is among the core symptoms of autism spectrum disorders (ASD). Despite evidence for altered excitation-inhibition balance contributing to states of hyper- or hypo-excitability in ASD, findings have been mixed. In this study, we used magnetic resonance spectroscopy (MRS) in a large sample of children with ASD and typically developing children to measure levels of GABA and glutamate+glutamine (Glx). Elevated sensorimotor Glx levels in ASD correlated with vibrotactile frequency discrimination thresholds and caregiver-reported hyper- and hyporesponsivity to sensory stimuli, symptoms that are central to ASD. These results provide support for the excitation-inhibition imbalance theory of ASD.
Introduction
Autism spectrum disorders (ASD) are primarily defined by impairments of social and communicative functioning. Abnormal patterns of sensory perception and responsivity are frequently reported for individuals with ASD. Indeed, sensory symptoms emerge in early development and remain pervasive across a diagnosed individual’s lifespan. The body of evidence suggesting that sensory abnormalities are related to altered excitation-inhibition balance is growing, with the original hypothesis suggesting that individuals with ASD have a more excitable sensory system, resulting in noisier and less efficient encoding of sensory stimuli (1). In light of this expanding evidence, sensory abnormalities are now recognized as core physiologic feature of ASD (2). Magnetic resonance spectroscopy (MRS) has frequently been used to measure levels of GABA and glutamate+glutamine (Glx) as indirect indices of inhibition and excitation, but results in ASD remain mixed due to heterogeneous methodology (3,4), and the extent to which GABA and Glx measures relate to sensory impairment remains unclear.In this study, we used edited MRS at 3T to measure GABA and Glx levels in a large sample of children with ASD and typically developing children (TDC). We further quantified sensory sensitivity and responsivity using vibrotactile psychophysical experiments and the Sensory Experiences Questionnaire, respectively
Methods
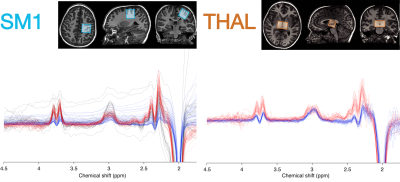
93 children with ASD (5 females; age 10.2 ± 1.6 y) and 98 TDC (23 females; age 9.7 ± 1.2 y) participated in this study. Psychophysical perceptual thresholds (e.g. detection, frequency discrimination, order judgement) were determined with a vibrotactile stimulation device, delivering stimuli to the index and middle finger of the left hand (5). Autism-Diagnostic Observation (ADOS-2) and Sensory Experiences Questionnaire (SEQ) were used to characterize symptom severity and caregiver-provided assessment of sensory responsivity.T1-weighted anatomical images (MP-RAGE) and edited MRS data were acquired on a Philips Achieva 3T scanner spanning 3 acquisition phases: 1) macromolecule-suppressed MEGA-PRESS, editing pulses at 1.9/1.5 ppm (6); 2) macromolecule-suppressed MEGA-PRESS with prospective frequency correction (7); 3) HERMES (editing pulses at 1.9/4.56 ppm) for simultaneous detection of GABA+macromolecules (GABA+) and glutathione (8). Shared acquisition parameters were: TR/TE = 2000/80 ms; 2 kHz spectral width; 2048 samples; including water reference. Figure 1 shows the MRS voxels measuring 30×30×30 mm3 over the right sensorimotor cortex (SM; phases 1-3) and 40×26×24 mm3 over the bilateral thalamus (THAL; phases 2-3). Data were analyzed using Gannet v3.1.3 (9). Metabolites were quantified from the difference spectra relative to creatine (“GABA/Cr”; “Glx/Cr”) and tissue water using alpha tissue correction in institutional units (“GABA”; “Glx”) (10).
Metabolite estimates from all acquisition phases were pooled using a linear-mixed effects model including age and sex as fixed factors and acquisition phase as a random factor (to reconcile systematic differences in editing efficiency and metabolite estimation between edited MRS methods). Residuals from this model were compared between ASD and control groups using linear models with participant as random factor. Correlation analyses were performed between metabolite levels, perceptual thresholds, and ADOS/SEQ scores.
Results
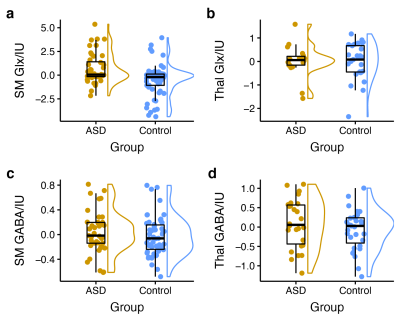
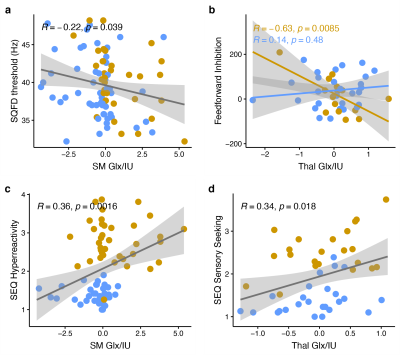
Edited spectra (color-coded by acquisition phase) are shown in Figure 1. There was a significant main effect of group on Glx levels [F(1, 146) = 12.27, p < 0.001], as well as a region ´ group interaction effect on Glx levels. Subsequent simple main effect analyses showed an effect of group for Glx in the sensorimotor voxel [higher in ASD; F(1,101) = 13.19, p < .001], but not in the thalamus voxel [F(1, 45) = 0.00, p = 0.945], while there was no effect of group for GABA in either region (Figure 2). These result patterns were also found for Glx/Cr and GABA/Cr, suggesting results were not driven by the reference compound.Figure 3 shows results from correlation analyses. Collapsed across groups, sequential frequency discrimination was linearly associated with sensorimotor Glx (r = -0.22, p = 0.039). A diagnostic dissociation was found for the relation between thalamic Glx and feedforward inhibition, showing no correlation in in TDC (r = 0.14, p = 0.48), but a strong negative relationship in children with ASD (r = -0.63, p = 0.009).
No associations were found between metabolite levels and ADOS scores.
Discussion
Higher sensorimotor Glx levels in ASD are consistent with the notion of reduced cortical excitation-inhibition balance. In addition, we found evidence for associations between Glx levels and markers of sensory processing impairment (both at the perceptual and reactive level, i.e., vibrotactile task performance and caregiver-reported scores of sensory responsivity). These findings support a potential link between local metabolite levels and severity of core autistic traits, specifically, altered tactile perception.Furthermore, our data suggest a different role of sensory gating in ASD (through thalamic Glx). Interestingly, we were not able to replicate previous findings (including our own work) of reduced sensorimotor GABA levels, including associations with vibrotactile task performance, despite a large sample size.
Conclusion
Higher sensorimotor Glx levels in children with ASD and their associations with altered tactile perception and sensory reactivity support the hypothesis of altered excitation-inhibition balance underlying sensory abnormalities in ASD.Acknowledgements
This work has been supported by NIH grants R21MH098228, R01MH106564, R00MH107719, and K99AG062230. JLH and NAP received salary support from the Nancy Lurie Marks Family Foundation as part of Autism Research Sensory Consortium. This study applies tools developed under NIH R01EB016089 and P41EB015909.References
1. Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267 doi: 10.1034/j.1601-183X.2003.00037.x.
2. Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J. Child Psychol. Psychiatry 2005;46:1255–1268 doi: 10.1111/j.1469-7610.2005.01431.x.
3. Ajram LA, Pereira AC, Durieux AMS, Velthius HE, Petrinovic MM, McAlonan GM. The contribution of [1H] magnetic resonance spectroscopy to the study of excitation-inhibition in autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019;89:236–244 doi: 10.1016/j.pnpbp.2018.09.010.
4. Ford TC, Crewther DP. A Comprehensive Review of the 1H-MRS Metabolite Spectrum in Autism Spectrum Disorder. Front. Mol. Neurosci. 2016;9 doi: 10.3389/fnmol.2016.00014.
5. Puts NAJ, Edden RAE, Wodka EL, Mostofsky SH, Tommerdahl M. A vibrotactile behavioral battery for investigating somatosensory processing in children and adults. J. Neurosci. Methods 2013;218:39–47 doi: 10.1016/j.jneumeth.2013.04.012.
6. Edden RA, Puts NA, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn Reson Med 2012;68:657–661 doi: 10.1002/mrm.24391.
7. Edden RAE, Oeltzschner G, Harris AD, et al. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J. Magn. Reson. Imaging 2016;44:1474–1482 doi: 10.1002/jmri.25304.
8. Saleh MG, Oeltzschner G, Chan KL, et al. Simultaneous edited MRS of GABA and glutathione. NeuroImage 2016;142:576–582 doi: 10.1016/j.neuroimage.2016.07.056.
9. Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 2014;40:1445–1452 doi: 10.1002/jmri.24478.
10. Harris AD, Puts NAJ, Edden RAE. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 2015;42 doi: 10.1002/jmri.24903.
Figures