2249
Mapping developmental trajectories of the cortex and its adjacent white matter for preterm neonates using DTI1USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA, United States, 2Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States
Synopsis
The brain development of cyto- and myeloarchitecture undergoes rapid and intensive changes during the third trimester. Diffusion tensor imaging (DTI) probes the microstructural organization of brain tissue by measuring the three-dimensional spatial diffusion of water molecules1. Using mean diffusivity (MD) and fractional anisotropy (FA), we show the spatiotemporal pattern of cortical and superficial white matter maturation in preterm neonates. This result might be useful in the prediction of neurodevelopmental outcome and functional impairments in preterm survivors.
Synopsis
The brain development of cyto- and myeloarchitecture undergoes rapid and intensive changes during the third trimester. Diffusion tensor imaging (DTI) probes the microstructural organization of brain tissue by measuring the three-dimensional spatial diffusion of water molecules1. Using mean diffusivity (MD) and fractional anisotropy (FA), we show the spatiotemporal pattern of cortical and superficial white matter maturation in preterm neonates. This result might be useful in the prediction of neurodevelopmental outcome and functional impairments in preterm survivors.Introduction
The cyto- and myeloarchitectural of the cerebral cortex and adjacent white matter undergoes major changes during the third trimester as a result of important developmental processes in dendritic arborization, glial proliferation, differentiation of radial glia, and synapse formation. DTI provides in vivo examinations of the microstructural development in cerebral organization2. The properties of water diffusion computed from DTI can not only be used to characterize white matter maturation in the neonatal population, but also offer insights into the development of the cerebral cortex3. The most commonly used DTI-based metrics are mean diffusivity (MD), and fractional anisotropy (FA)4. It has been observed that MD decreases during maturation because the water content is reduced due to increasing neuron density and microstructure complexity5. In contrast, cortical FA decreases due to continuous radial glia progression, the arrival of axons, intracortical axonal premyelination, and basal dendritic arborization4 6. Using DTI metrics, we aimed to characterize the complex cyto- and myeloarchitectural changes of the cerebral cortex2 and adjacent superficial white matter (sWM) during the critical periods of development.Methods
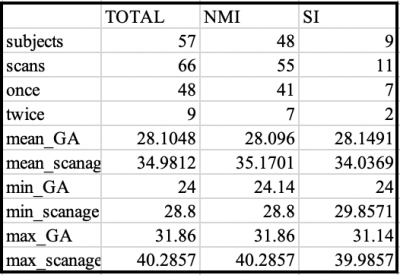
Our dataset included 57 preterm infants (Table 1), who underwent 66 MR scanning as soon as they were clinically stable and prior to discharge from the hospital (postmenstrual age (PMA): 28-41 weeks). Diffusion-weighted images were obtained using a single-shot echo-planar sequence: 30 non-collinear diffusion gradients with a b-value of 1000s/mm2, one non-diffusion weighted (b0) volumes, echo/repetition time = 80/8000ms, voxel size = 2×2×2 mm3, acquisition time = 4:08 min. DTI images were denoised, to enhance the images against motion artifacts, and masked. In this study, we use the surface-based parcellation, in which vertices that belong to the same anatomical structure are grouped. Here we used the automatic anatomical labeling parcellation7 mapped to the neonatal cortical template using the method described in NEOCIVET8 to retrieve cortical and sWM regions of interest (ROIs). 81,924 vertices sampled on each individual surface were labeled into 76 ROIs. We then calculated the mean of each DTI parameters for each ROI to analyze the pattern of spatiotemporal brain maturation. Finally, we used vertex-wise linear fitting models to correlate MD and FA values with PMA at scan while gestational age at birth and sex were corrected.Result
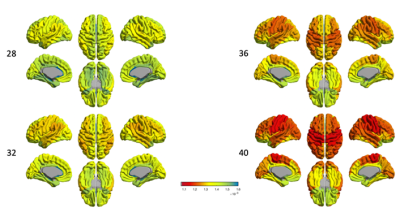
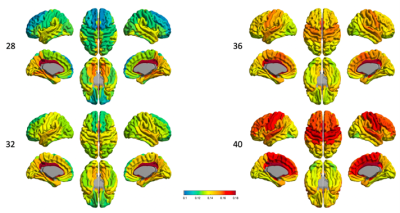
DTI-MDIn the cortex (Fig.1), the dorsal and lateral cortical convexities presented consistently lower mean diffusivity (MD) than other cortical regions throughout the 3rd trimester. The medial and basal cortical regions, including olfactory, basal/medial temporal, orbitofrontal and cingulate cortices, recorded higher MD values during the 3rd trimester. In sWM (Fig.2), the degree of MD at sWM voxels sampled 2mm below from the GM/WM boundary differed from that seen in the cortical GM. However, the overall spatiotemporal pattern of MD in sWM was very similar to the changes in cortical GM.
DTI-FA
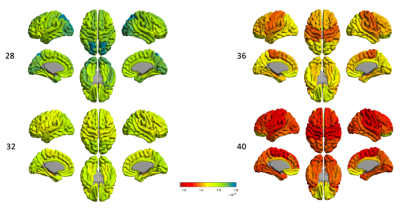
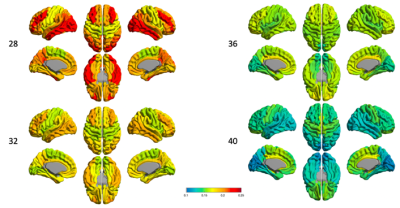
At 28 week of PMA (Fig.3), FA values in lateral frontal, lateral temporal, and lateral occipital cortical GM regions are relatively high and relatively low in the primary sensorimotor areas. However, we found the transition to the opposite pattern by 40 GW, in which the frontal, temporal, and occipital regions showed rapidly decreased FA values by 40 GW.The FA value increased over PMA in sWM (Fig.4), but different regions had distinguished development pattern. The cingulate region remains relatively high FA value throughout different PMAs during the 3rd trimester (Fig.4), the frontal lobe especially the superior frontal part has lowest FA value at 28GW compare to other sWM brain regions.
Discussion
During brain maturation, cortical radial architecture is disrupted by the basal dendrites, synaptic formation and intracortical axonal myelination2 9, which may lead to decreases in FA that were observed in our preterm neonatal data. MD is thought to negatively correlate with neuron density2 and microstructure complexity2. Lower MD overtime during the 3rd trimester was indeed found in the central and lateral regions of cortical GM. The maturation of sWM was observed as increases in FA and decreases in MD in our data. This maturation process may involve the expansion in the number of premature oligodendrocytes, development of the cytoskeleton2 and the myelination that begins around the late 3rd trimester. However, we observed that the aforementioned processes are asynchronous among different brain regions during maturation. Our finding of spatiotemporally changing FA in sWM indicates highly intensive microstructural changes in the frontal, central, and parietal regions during early brain development. MD changed more rapidly over time in the ventral part of sWM except the orbital region. The discordance of the spatiotempral patterns of FA and MD in sWM needs a further study to clarify. Interferences of the neurodevelopment due to perinatal brain injury and postnatal clinical risk factors may affect the pattern we found, leading to impairment of cognition and neuromotor function in preterm survivors10.Acknowledgements
No acknowledgement found.References
1. Basser, P.J., J. Mattiello, and D. LeBihan, MR diffusion tensor spectroscopy and imaging. Biophysical journal, 1994. 66(1): p. 259-267.
2. Yoshida, S., et al., Diffusion tensor imaging of normal brain development. Pediatric radiology, 2013. 43(1): p. 15-27.
3. Huang, H., et al., White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage, 2006. 33(1): p. 27-38.
4. Mukherjee, P., et al., Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. American Journal of Neuroradiology, 2002. 23(9): p. 1445-1456.
5. Almli, C.R., et al., The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. Neuroimage, 2007. 35(1): p. 308-325.
6. Aeby, A., et al., Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. Neuroimage, 2013. 78: p. 145-151.
7. Tzourio-Mazoyer, N., et al., Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 2002. 15(1): p. 273-289.
8. Kim, H., et al., NEOCIVET: Towards accurate morphometry of neonatal gyrification and clinical applications in preterm newborns. NeuroImage, 2016. 138: p. 28-42.
9. Ouyang, M., et al., Differential cortical microstructural maturation in the preterm human brain with diffusion kurtosis and tensor imaging. Proceedings of the National Academy of Sciences, 2019. 116(10): p. 4681-4688.
10. Khwaja, O. and J. Volpe, Pathogenesis of cerebral white matter injury of prematurity. Archives of Disease in Childhood-Fetal and Neonatal Edition, 2008. 93(2): p. F153-F161.