2246
High resolution diffusion imaging in the unfixed post-mortem neonatal brain1Wellcome Centre for Integrative Neuroimaging, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Department of Paediatrics, University of Oxford, Oxford, United Kingdom, 3Newborn Care Unit, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom, 4Sir Peter Mansfield Imaging Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 5NIHR Biomedical Research Centre, University of Nottingham, Nottingham, United Kingdom, 6Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 7Department of Radiology, Harvard Medical School, Boston, MA, United States, 8Department of Radiology, University of Chicago, Chicago, IL, United States, 9Imaging Centre of Excellence, College of Medical, Veterinary & Life Sciences, University of Glasgow, Glasgow, United Kingdom, 10Department of Biomedical Engineering, School of Biomedical Engineering and Imaging Sciences, King’s College London, King’s Health Partners, St. Thomas’ Hospital, London, United Kingdom, 11Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, King’s Health Partners, St. Thomas’ Hospital, London, United Kingdom, 12Department of Psychiatry, University of Oxford, Oxford, United Kingdom
Synopsis
In this work, we develop a 7T experimental platform to acquire high resolution diffusion MRI images in unfixed post-mortem infant brains. Here we present results from a first infant. The post-mortem structural images demonstrate superior SNR and improved depiction of small structures than age-matched low-resolution in vivo data. The post-mortem diffusion MRI images show sufficient SNR to support the considerably smaller voxel volume compared to in vivo. The high spatial resolution of the post-mortem data enables the depiction of brain features (e.g., cortical radiality, subplate organization) that are less prominent in low-resolution in vivo data.
Introduction
Diffusion MRI (dMRI) of the neonatal brain allows visualisation of the organisational structure of maturing fibres and improves our understanding of human brain development, but requires high resolution to assess small structures. Post-mortem dMRI has the potential to achieve higher resolution than that in vivo via long scan times. Previous studies have reported high-resolution dMRI data of fixed neonatal brains1-4. Alterations to fixed tissue properties are often detrimental to dMRI measurements5 and complicate interpretation of the results. Consequently, we have developed a 7T platform to acquire high-resolution dMRI data in unfixed post-mortem infant brains shortly after death, as part of the developing human connectome project (http://www.developingconnectome.org).Methods
We present results from a first infant, born at 23+0 weeks' gestation and passed away at 29+4 weeks' gestation. The infant was scanned overnight, accompanied by an experienced neonatal nurse, in transit between the neonatal unit and the mortuary, using a Siemens 7T scanner with a custom post-mortem neonate coil (2TX/16RX, Rapid biomedical). Active cooling of the infant was used, which is crucial to maintain a low, stable body temperature in the absence of autoregulation and presence of RF absorption.dMRI was acquired with simultaneous multi-slice spin-echo with readout-segmented EPI and monopolar diffusion preparation6 at 0.8mm isotropic resolution. Scan parameters: TR/TE=10.8s/113ms, FOV=150x150x104mm3, GRAPPA=2, Multiband=2, 7 segments, echo spacing=0.4ms, b=3000/6000/9000s/mm2 (64/88/128 directions), 21 b=0 volumes, scan time=6h50min. T2w structural images were acquired at 0.4mm isotropic resolution: 3D-SPACE, TR/TE=2300/586ms, FOV=155x155x96mm3, 6 repetitions, scan time=58min. For comparison, images of five age-matched infants from the in vivo dHCP study at 3T were also presented.
The T2w image was used to reconstruct the cortical surface7. dMRI images were distortion corrected8 and then analysed to extract: (i) diffusion tensor/kurtosis measures9, (ii) NODDI parameters10, and (iii) neonate-specific automated tractography11.
Results
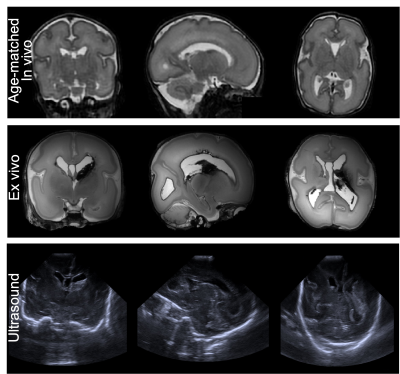
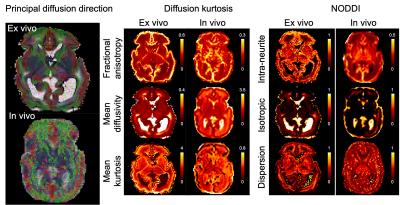
Structurals: Fig.1 shows the post-mortem T2w images alongside an age-matched in-vivo infant. The post-mortem data demonstrate higher SNR and improved depiction of small structures. The post-mortem infant had an intraventricular haemorrhage, which can be seen on cranial ultrasound images taken prior to death but are more clearly visible on the post-mortem T2w images.dMRI overview: Fig.2 demonstrates the post-mortem data have sufficient SNR to support the 6.6-fold smaller voxel volume compared to in-vivo. The overall spatial patterns of diffusion-derived metrics are consistent with in-vivo data, suggesting good quality fitting. Nevertheless, there are notable differences in dMRI-derived values. Our post-mortem data exhibited higher intra-neurite fraction and mean kurtosis than the in vivo data (Fig.2,4), which would be consistent with cell swelling in the first 24-hours after death. Post-mortem mean diffusivity is also considerably lower than that of the in-vivo data, probably a combined effect of lower temperature and microstructural changes.
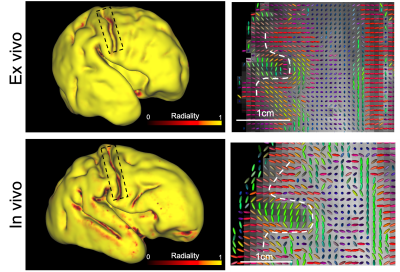
Cortical radiality: Fig.3 shows a radiality index (0=tangential,1=radial) on the cortical surface. The principal diffusion directions are observed to be predominately radial with respect to the cortical surface (also shown in Fig.2), consistent with organisation of the cortical plate at this gestational age12. Most of cortex in the post-mortem data has a radiality=1, whereas the in-vivo data has lower radiality in many regions. This likely reflects partial volume with neighbouring white matter. The in-vivo data show this effect in regions where white matter fibres abut radial grey matter at 90°: on gyral banks where fibres turn to enter, and in gyral fundi next to U-fibres. A similar partial volume effect is seen in the post-mortem data, but is largely confined to the banks of the central sulcus.
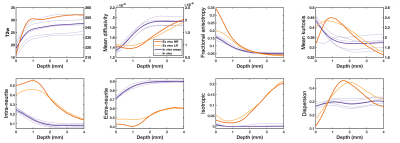
Subplate: Fig.4 shows T2w image intensity and diffusion parameters plotted as a function of the distance from the cortical plate (using FSL’s distancemap command13,14 where cortical voxels have distance=0 and other voxels have a higher distance value with a larger distance from the cortex). The post-mortem T2w profile rises then falls at a depth of ~1mm, which corresponds to the superficial subplate layer15-17. The post-mortem dMRI data (red) show corresponding features at the same depth for intra-neurite fraction and mean kurtosis (peak at 1mm), and an inverted pattern in extra-neurite fraction and mean diffusivity (trough at 1mm). This pattern might reflect the synapse-rich environment of superficial subplate layer16. This pattern is not observed in the lower-resolution in vivo data (purple). Downsampling the post-mortem data to lower resolution (yellow) reduced the prominence of these subplate features but not eliminate it, suggesting influence from both resolution and microstructural changes.
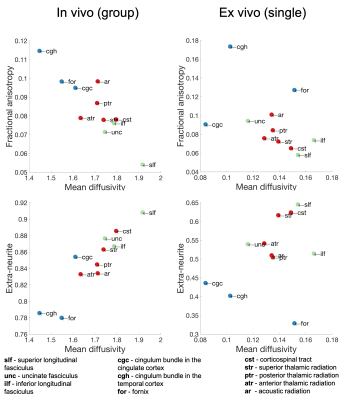
White matter tracts: Fig.5 shows an exploratory evaluation of three categories of white matter pathways: association (green), limbic (blue) and projection (red). Both in-vivo and post-mortem data were able to largely separate these different categories based on pairings of dMRI-derived measures, in particular by combining mean diffusivity with either fractional anisotropy or the extra-neurite fraction. Notably, the single post-mortem dataset exhibits similar organisation as the in-vivo group analysis across n=5 infants of similar gestational age.
Conclusions
Preliminary results using our high-resolution post-mortem neonatal imaging platform demonstrate the ability to resolve features that are less prominent in lower-resolution in vivo data. Future work will collect a cohort of scans at a range of gestational ages that will form an open resource for comparison with in-vivo data and development of a post-mortem atlas across early neonatal life to inform brain development.Acknowledgements
RS and KM contributed equally to this work. The research leading to these results has received funding from the European Research Council under the European Union Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 319456. We are grateful to the families who generously supported this trial. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). WW is supported by the Royal Academy of Engineering (RF\201819\18\92). KM is supported by the Wellcome Trust (WT202788/Z/16/A). LB, FA, REF, VM, FM, and RS are supported by the Wellcome Trust (207457/Z/17/Z).
References
1. Huang, H., Zhang, J., Wakana, S., Zhang, W., Ren, T., Richards, L.J., Yarowsky, P., Donohue, P., Graham, E., van Zijl, P.C.M., Mori, S., 2006. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage 33, 27–38.
2. Huang, H., Xue, R., Zhang, J., Ren, T., Richards, L.J., Yarowsky, P., Miller, M.I., Mori, S., 2009. Anatomical Characterization of Human Fetal Brain Development with Diffusion Tensor Magnetic Resonance Imaging. J. Neurosci. 29, 4263–4273.
3. Takahashi, E., Folkerth, R.D., Galaburda, A.M., Grant, P.E., 2012. Emerging cerebral connectivity in the human fetal brain: an MR tractography study. Cereb. Cortex N. Y. N 1991 22, 455–464.
4. Vasung, L., Charvet, C.J., Shiohama, T., Gagoski, B., Levman, J., Takahashi, E., 2019. Ex vivo fetal brain MRI: Recent advances, challenges, and future directions. NeuroImage 195, 23–37.
5. Roebroeck, A., Miller, K.L., Aggarwal, M., 2019. Ex vivo diffusion MRI of the human brain: Technical challenges and recent advances. NMR Biomed. 32, e3941.
6. Frost, R., Jezzard, P., Douaud, G., Clare, S., Porter, D.A., Miller, K.L., 2015. Scan time reduction for readout-segmented EPI using simultaneous multislice acceleration: Diffusion-weighted imaging at 3 and 7 Tesla. Magn. Reson. Med. 74, 136–149.
7. Makropoulos, A., Robinson, E.C., Schuh, A., Wright, R., Fitzgibbon, S., Bozek, J., Counsell, S.J., Steinweg, J., Vecchiato, K., Passerat-Palmbach, J., Lenz, G., Mortari, F., Tenev, T., Duff, E.P., Bastiani, M., Cordero-Grande, L., Hughes, E., Tusor, N., Tournier, J.-D., Hutter, J., Price, A.N., Teixeira, R.P.A.G., Murgasova, M., Victor, S., Kelly, C., Rutherford, M.A., Smith, S.M., Edwards, A.D., Hajnal, J.V., Jenkinson, M., Rueckert, D., 2018. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. NeuroImage 173, 88–112.
8. Andersson, J.L.R., Sotiropoulos, S.N., 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078.
9. Jensen, J.H., Helpern, J.A., Ramani, A., Lu, H., Kaczynski, K., 2005. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 53, 1432–1440.
10. Zhang, H., Schneider, T., Wheeler-Kingshott, C.A., Alexander, D.C., 2012. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016.
11. Bastiani, M., Andersson, J.L.R., Cordero-Grande, L., Murgasova, M., Hutter, J., Price, A.N., Makropoulos, A., Fitzgibbon, S.P., Hughes, E., Rueckert, D., Victor, S., Rutherford, M., Edwards, A.D., Smith, S.M., Tournier, J.-D., Hajnal, J.V., Jbabdi, S., Sotiropoulos, S.N., 2019. Automated processing pipeline for neonatal diffusion MRI in the developing Human Connectome Project. NeuroImage 185, 750–763.
12. McKinstry, R.C., 2002. Radial Organization of Developing Preterm Human Cerebral Cortex Revealed by Non-invasive Water Diffusion Anisotropy MRI. Cereb. Cortex 12, 1237–1243.
13. Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens, T.E.J., Johansen-Berg, H., Bannister, P.R., De Luca, M., Drobnjak, I., Flitney, D.E., Niazy, R.K., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J.M., Matthews, P.M., 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, Mathematics in Brain Imaging 23, S208–S219.
14. Jenkinson, M., Beckmann, C.F., Behrens, T.E.J., Woolrich, M.W., Smith, S.M., 2012. FSL. NeuroImage, 20 YEARS OF fMRI 62, 782–790.
15. Pogledic, I., Kostovic, I., Fallet-Bianco, C., Adle-Biassette, H., Gressens, P., Verney, C., 2014. Involvement of the Subplate Zone in Preterm Infants with Periventricular White Matter Injury: Subplate in Periventricular White Matter Injury. Brain Pathol. 24, 128–141.
16. Kostović, I., Išasegi, I.Ž., Krsnik, Ž., 2019. Sublaminar organization of the human subplate: developmental changes in the distribution of neurons, glia, growing axons and extracellular matrix. J. Anat. 235, 481–506.
17. Pogledic, I., Schwartz, E., Mitter, C., Baltzer, P., Milos, R.-I., Gruber, G.M., Brugger, P.C., Hainfellner, J., Bettelheim, D., Langs, G., Kasprian, G., Prayer, D., 2020. The Subplate Layers: The Superficial and Deep Subplate Can be Discriminated on 3 Tesla Human Fetal Postmortem MRI. Cereb. Cortex bhaa099.
Figures