2243
Optimizing Brain Injury Biomarkers in a piglet model of Neonatal Encephalopathy: combining perfusion with proton MRS1Brain Repair and Rehabilitation, Institute of Neurology, UCL Queen Square Institute of Neurology, London, United Kingdom, 2Medical Physics and Biomedical Engineering, UCLH NHS Foundation Trust, London, United Kingdom, 3Neonatology, UCL EGA Institute for Women's Health, London, United Kingdom, 4UCL Queen Square Institute of Neurology, London, United Kingdom
Synopsis
Despite therapeutic hypothermia, some survivors of neonatal encephalopathy (NE) still have adverse outcomes. Surrogate outcome measures or biomarkers are needed for early detection of babies with adverse outcome so that adjunct therapies can be considered. Basal ganglia and thalamus (BGT) lactate/ N-Acetyl-Aspartate (Lac/NAA) peak area ratio acquired with proton magnetic resonance spectroscopy (MRS) is a reliable predictor of developmental outcome at two years. Cerebral blood flow (CBF) changes in parallel with the metabolic changes after hypoxia ischaemia (HI). We hypothesised that the combination of CBF with BGT Lac/NAA would be more closely associated with quantitative cell death than either alone.
Background
Surrogate outcome measures or biomarkers are needed for early detection of babies with adverse outcome so that adjunct therapies can be considered. Basal ganglia and thalamus (BGT) lactate/ N-Acetyl-Aspartate (Lac/NAA) peak area ratio acquired with proton magnetic resonance spectroscopy (MRS) is a reliable predictor of developmental outcome at two years1. Following severe perinatal hypoxia ischaemia HI, initial hypoperfusion is followed by hyperperfusion in a predictable pattern. This increased cerebral blood flow (CBF) (also termed luxury perfusion or hyperperfusion) on day 2–3 reflects a state of maximal vasodilation in response to the release of vasoactive factors and is associated with adverse neurodevelopmental outcome2 even with HT. One study in babies with of neonatal encephalopathy (NE) demonstrated some improvement in adverse outcome prediction using a combination of Lac/NAA with CBF measurement by Arterial Spin Labelling (ASL)3. A clearer understanding of the association between imaging/spectroscopy biomarkers and immunohistochemistry (IHC) would help with outcome prediction and clinical translation of experimental neuroprotective therapies. In this work, a piglet model of HI and inflammation sensitized HI was used to examine the links between CBF and IHC and to compare the predictive value of combined CBF and Lac/NAA biomarkers at two timepoints after injury.Methods
Subjects: 46 male sedated, anesthetized and mechanically ventilated piglets were subjected to hypoxia-ischemia. Animals were treated as per standard clinical guidelines and were scanned on a 3T Philips Achieva System (Philips Medical Systems, Best, The Netherlands) in a custom designed incubator for MRI/S at 24h and 48h post injury. Proton MRS was acquired with 8 × 8 matrix and 8 × 8 × 10 mm3 voxels with TR/TE 2000ms/288 ms. MRS data for the left basal ganglia – thalamus (BGT) and white matter (WM) voxels were selected and processed using Tarquin with threonine included in the basis set1. Lac/NAA was calculated from the amplitude of the fitted components (Lac+Thr/NAA+NAAG). Pseudocontinuous ASL (pCASL) parameters were: 2D EPI readout, TR/TE 10ms/4500ms, labelling duration: 1800ms, post labelling delay: 2000ms, voxel size: 2.5 x 2.5mm, slice thickness 4mm, 12 slices, SENSE 2.3, fat and background suppression, 60 control-label pairs, PD image for calibration (TR of 9000ms). T2 structural scans were also acquired. CBF quantification was performed in line with recommendations4 using blood T1 of 2.1s based on mean haematocrit value5 and labelling efficiency (α)=0.8 computed using simulator6 based on velocities measured at the level of the labelling. Blood-brain partition coefficient λ was set to 15. Region of interest (ROI) analysis: ROIs were identified automatically by atlas7 labels propagation. First, structural template scans were co-registered to each piglet structural scan that was resampled to isotropic voxel size. ASL data and structural scans were then co-registered and combination of transformations used to propagate and down sample labels. Finally, CBF was calculated within BGT, cortical and whole brain regions at two timepoints and mean of the twoHistology: TUNEL-positive cells, Iba1 ramification index and cleaved caspase 3 were counted. Statistical analysis: relationships were assessed using Pearson’s correlation and prediction was done using regression analysis (STATA/SE 15.1 software). Single and multiple linear regression was used to determine the prediction of (TUNEL-positive) cell death in the BGT by the CBF and Lac/NAA separately and in combination, respectively. Regression analyses was used with 24h and 48h CBF in the BGT to assess their separate predictions of cell death in both BGT and whole brain (WB).Results
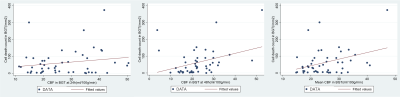
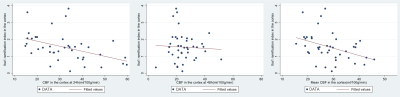
Mean CBF showed best significant positive correlation with TUNEL-positive cells (r = 0.36, p = 0.01 in the BGT at 48h (figure1), and best negative correlation with Iba1 ramification index in the cortex at 24h (r = -0.44, p < 0.01) and (r = -0.39, p = 0.01) (figure 2). Lac/NAA correlated best with IHC markers. Simple linear regression analysis shows that CBF in the BGT can predict 13% (r2 = 0.13, p = 0.01) of cell death, less than the prediction power of Lac/NAA, 16% (r2 = 0.16, p < 0.01). Multiple regression shows that the combination of both offers better prediction (r2 = 0.29), but this is not statistically significant (p = 0.69).Discussion and Conclusion
The correlation between increased CBF and TUNEL-positive cells is consistent with the observed hyperperfusion on day 2-3 associated with adverse outcome in babies with NE and reflects a state of maximal vasodilation in response to the increased release of vasoactive factors in the most severely affected parts of the brain. CBF is likely to change more rapidly over days 2-3 than Lac/NAA which was more strongly associated with cell death than CBF. With Lac/NAA, the combined increase in lactate and reduced NAA on MRS (translating to a high Lac/NAA peak area ratio) reflects brain mitochondrial impairment and impaired oxidative metabolism during “secondary energy failure”8. We conclude that, while BGT CBF is associated with cell death in BGT at 48h, Lac/NAA peak area ratio is more stable, more closely associated with cell death and a validated outcome marker in babies with NE. The combination of both parameters does not improve prediction of cell death.Acknowledgements
These studies were funded by the Medical Research Council MR/M006743/1, Chiesi Pharmaceuticals (research grant), and Action Medical Research for Children (GN2295). This work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the UK Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme.References
[1] Mitra S et all. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed 2019;104: F424–F432.
[2] Wintermark P et all Temporal evolution of MR perfusion in neonatal hypoxic-ischemic encephalopathy. JMagn Reson Imaging 27(6):1229–1234 2008
[3] De Vis J.B Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur Radiol (2015) 25:113–12
[4] Alsop D.C et all. Recommended Implementation of Arterial Spin-Labeled Perfusion MRI for Clinical Applications: A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magnetic Resonance in Medicine 2015; 73:102–116.
[5] Varela M et all A method for rapid in vivo measurement of blood T1. NMR in biomedicine, vol. 24, pp. 80–8, Jan. 2011.
[6] Sokolska et all Effect of labelling plane angulation and position on labelling efficiency and cerebral blood flow quantification in pseudo-continuous arterial spin labelling Magnetic resonance imaging 59, 61-67 2019
[7] Conrad MS et all An in vivo three-dimensional magnetic resonance imaging-based averaged brain collection of the neonatal piglet. PLoS ONE. (2014) 9:e107650.
[8] Peng R et all. Proton Magnetic Resonance Spectroscopy Lactate/N-Acetylaspartate Within 48 h Predicts Cell Death Following Varied Neuroprotective Interventions in a Piglet Model of Hypoxia–Ischemia With and Without Inflammation-Sensitization Front. Neurol., 04 September 2020
Figures