2241
Relationship between brain temperature and prognosis during hypothermia in newborns with hypoxic-ischemic encephalopathy1Department of Molecular Imaging and Theranostics, National Institute for Quantum and Radiological Science and Technology, Chiba, Japan, 2Department of Radiology, Kanagawa Children’s Medical Center, Yokohama, Japan, 3Department of Neonatology, Kanagawa Children’s Medical Center, Yokohama, Japan, 4Institute of Applied Physics, University of Tsukuba, Tsukuba, Japan
Synopsis
We examined brain temperatures of 81 neonates with hypoxic-ischemic encephalopathy during and/or after hypothermia therapy. The brain temperature was obtained from magnetic resonance spectroscopy data (3T). The subjects also had a neurological developmental test at the age of 18–22 months, and were divided into favorable and adverse outcome groups. Brain temperatures for each outcome were significantly lower during hypothermia compared with those after hypothermia. Although the poor prognosis group tended to have a large dispersion of brain temperature after hypothermia, there was no significant difference in brain temperature between the favorable and adverse outcome groups.
Introduction
Hypothermia therapy for neonates with hypoxic-ischemic encephalopathy (HIE) is performed to protect against neurodegeneration by reducing the metabolic rate and oxygen consumption during brain encephalopathy, which improves the chance of survival and reduces disability [1-5]. White matter and deep gray matter abnormalities, such as impaired microstructure and metabolism, are strongly associated with prognosis in neonates with HIE [6-8], and higher brain temperatures were observed in cases with severe HIE during and after hypothermia compared with those with moderate HIE [3]. Therefore, the purpose of our study was to investigate whether brain temperature in neonates with HIE during and after hypothermia therapy was associated with their prognosis.Methods
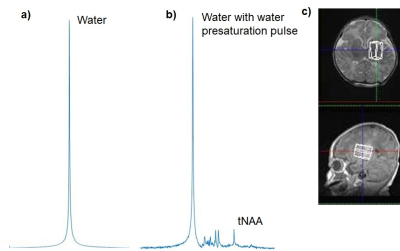
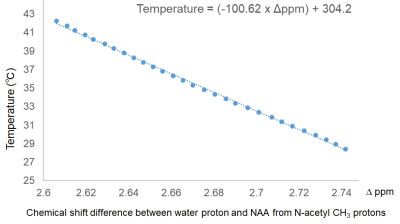
This was a retrospective study. Eighty-one neonates with HIE were enrolled. They received hypothermia therapy for 72 hours, and had magnetic resonance spectroscopy (MRS) of the deep gray matter and centrum semiovale during and/or after hypothermia on postnatal days 0–3 and 6–12, respectively. The parameters of single-voxel proton MRS (3T, Siemens, Germany) were as follows: PRESS sequence [9], TE/TR=30/5000 ms, observation bandwidth = 2000 Hz, data points = 1024, excitations = 6–23 and 2 for PRESS sequence with and without water presaturation pulse, respectively. Volumes of interest in the deep gray matter and centrum semiovale were 2.9–7.1 mL and 2.5–7.7 mL, respectively. Using in-house software running in MATLAB (MathWorks, USA), the time domain MRS data was zero-filled to 2048 to data points and fast-Fourier transformed into an absolute spectrum. MRS data with a signal-to-noise ratio of 4 or less were excluded in this study. The brain temperatures were calculated using the formula (−100.62 × Δppm) + 304.2 (1) for each MRS data, where Δppm was the chemical shift difference between N-acetylaspartate and N-acetylaspartylglutamate peak and the water peak, obtained from samples with and without water presaturation pulse, respectively (Fig. 1). Equation (1) was obtained using an NMR spectrometer (Fig. 2; 400MHz, Jeol, Tokyo, Japan). Frequency drift between a pair of spectra was corrected before the temperature calculation. Subjects who survived neonatal intensive care unit hospitalization were neurodevelopmentally evaluated at the age of 18–22 months using the Kyoto Scale of Psychologic Development (KSPD) scale. We defined adverse outcomes as a KSPD developmental quotient score less than 85, and Gross Motor Function Classification System grade 2 or over, epilepsy, or hearing impairment [26, 27]. Comparisons of brain temperatures between subjects with different prognoses and those during and after hypothermia were performed using the Mann–Whitney U-test. A p-value less than 0.05 was considered statistically significant.Results
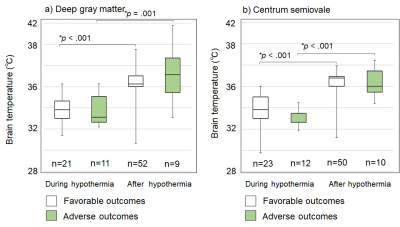
In this study, 18 of 81 neonates (22%) had adverse outcomes, and the remaining 63 neonates (78%) had favorable outcomes. Overall, 187 brain temperatures (93 deep gray matter, and 94 centrum semiovale) were obtained. Brain temperatures of both outcomes were significantly lower during hypothermia compared with after hypothermia (Fig. 3). Comparisons between favorable and adverse outcomes showed no significant differences in brain temperatures during or after hypothermia (Fig. 3) (favorable-outcomes during hypothermia: deep gray matter, 33.9±1.4°C [mean±standard deviation], and centrum semiovale, 34.7±1.7°C; and after hypothermia: deep gray matter, 36.3±1.5°C, and centrum semiovale, 37.6±1.2°C; adverse-outcomes, during hypothermia: deep gray matter, 33.8±1.5°C, and centrum semiovale, 34.3±1.2°C; and after hypothermia: deep gray matter, 37.3±2.5°C, and white matter, 38.3±1.6°C).Discussion
This retrospective study measured brain temperatures during and/or after hypothermia therapy for each of 81 neonates with HIE. Brain temperatures during hypothermia were significantly decreased compared with those after hypothermia, similar to previously reported studies [3-5]. However, there was no difference in brain temperature between outcomes with different prognoses during or after hypothermia (Fig. 3). Wu et al., reported significant differences in brain temperatures during and after hypothermia when comparing severe and mild HIE newborns, suggesting there was an enhanced inflammation cascade in severe HIE newborns [3]. Children with a poor prognosis may have had severe encephalopathy, but if the cerebral circulatory function diffuses heat generated in the brain to the body through the blood as is normal, elevated brain temperatures can be avoided [4]. This might have occurred in many of our study subjects. However, during adverse outcomes, the dispersion of brain temperature after therapy tended to be high (Fig. 3). A possible explanation is that some patients with adverse outcomes had a stronger inflammatory and/or circular system deficiency leading to a higher brain temperature, whereas others may have had a low brain temperature due to reduced energy production from oxygen and glucose. Further studies are needed to examine the symptoms of encephalopathy in each patient in detail.Conclusions
This study reports no correlation between HIE neonatal brain temperature during and after hypothermia and neurodevelopmental assessments at 18–22 months of age.Acknowledgements
The authors thank Dr Kikuko Hayamizu, and Tomoyuki Haishi for technical support, Mr Masahiko Sato, Mr Kohki Kusagiri, Mr Yasutake Muramoto, and Ai Kitagawa for help during acquisition of MR images and spectroscopy data, and Ms Kazumi Sato and Ms Akemi Suzuki for assistance with data analysis. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 15K09943 and19K08213].
References
1. Shankaran, S., et al., Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med, 2005; 353:1574-84.
2. Gluckman, P.D., et al., Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet, 2005; 365:663-70.
3. Wu, T.W., et al., Brain temperature in neonates with hypoxic-ischemic encephalopathy during therapeutic hypothermia. J Pediatr, 2014; 165(6):1129-34.
4. Wang, H., et al., Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front Neurosci, 2014; 8:307.
5. Owji, Z.P., et al., Brain Temperature Is Increased During the First Days of Life in Asphyxiated Newborns: Developing Brain Injury Despite Hypothermia Treatment. AJNR Am J Neuroradiol, 2017; 38: 2180-2186.
6. Martinez-Biarge, M., et al., White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr, 2012; 161:799-807.
7. Heursen, E.M., et al., Prognostic Value of the Apparent Diffusion Coefficient in Newborns with Hypoxic-Ischaemic Encephalopathy Treated with Therapeutic Hypothermia. Neonatology, 2017; 112: 67-72.
8. Shibasaki, J., et al., Changes in Brain Metabolite Concentrations after Neonatal Hypoxic-ischemic Encephalopathy. Radiology, 2018; 288: 840-848.
9. Bottomley, P.A., Spatial Localization in Nmr-Spectroscopy In vivo. Annals of the New York Academy of Sciences, 1987; 508:333-348.
Figures