2237
Ultra-high field MR spectroscopic imaging at 7 Tesla in Multiple Sclerosis: metabolic fingerprinting of iron rim lesions1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 3Department of Neurology, Medical University of Vienna, Vienna, Austria
Synopsis
Conventional T1/T2-weighted magnetic resonance imaging (MRI) is the method of choice for diagnosis and treatment monitoring of Multiple Sclerosis (MS). Susceptibility weighted imaging (SWI) provides additional information about iron deposition. In addition to these imaging modalities, MR Spectroscopic Imaging (MRSI) can detect pathologies on a biochemical level. In 32 relapsing remitting (RRMS) patients, we showed - with ultra-high spatial resolution Free Induction Decay(FID)-MRSI at 7T - the metabolic changes associated with different types of iron accumulation and the metabolic gradient spanning from within the lesion to its close proximity.
Introduction
In Multiple Sclerosis (MS), conventional T1/T2-weighted MRI is the method of choice for diagnosis and treatment monitoring. In contrast to T1/T2 imaging, which shows general macroscopic tissue damage, SWI can provide additional information about iron deposition in MS lesions. These iron accumulations have been linked to increased tissue damage [1], slow expansion of lesions [2] and worse clinical course [3].In addition to these imaging modalities, FID-MRSI is able to detect pathologies on a biochemical level, which provides further insights into underlaying pathological processes [4-5].
Decreased N-acetylaspartate (NAA) caused by axonal loss, elevated myoInositol (mIns) due to inflammation-induced glial activation and decreased creatine (Cr) reflecting mitochondrial dysfunction are the main metabolic hallmarks of MS lesions [6-7].
The objective of our study was to (1) categorize different iron deposition types at SWI, (2) compare metabolic changes of these groups and (3) investigate whether the metabolic levels were equally distributed over the whole lesion and its vicinity between lesions with and without iron accumulation.
Materials and Methods
After approval of the institutional review board, 32 RRMS patients (17 female/15 male; age 36.69±10.23 years) were scanned using a 7 T whole-body MR scanner (Magnetom; Siemens Healthcare, Erlangen, Germany) and a 32-channel head coil (NovaMedical, Wilmington, MA).Prior to spectroscopic data collection, 3D-MRI including T1-weighted MP2RAGE with a nominal resolution of 0.8mm3 isotropic, T2-weighted 3D-FLAIR with a nominal resolution of 0.86mm3 isotropic, and SWI (nominal resolution of 0.3×0.3×1.2mm3; TE=25ms) were obtained.
FID-MRSI was performed with TR/acquisition delay (AD), 200/1.3ms; FOV, 220×220mm²; matrix size, 100×100; slice thickness, 8mm; flip angle, 29°; spectral bandwidth, 3kHz; 1024 samples; WET water suppression; 4-fold 2D-CAIPIRINHA acceleration; acquisition time, 6:06min [8].
In-house developed MATLAB post-processing routine was performed including MUSICAL coil combination [5], 2D-CAIPIRINHA reconstruction [8], spatial Hamming filtering and lipid signal removal via L2-regularization [9]. The spectra were fitted with LCModel in the spectral range of 1.8-4.2 ppm using a simulated basis-set consisting of 17 metabolites and a measured macromolecular background [10]. Metabolic maps, quantification precision (Crámer-Rao Lower Bounds (CRLBs)) and spectral quality (SNR, linewidth) were created.
After resampling MP2RAGE images to slices matching the respective MRSI slice, “black hole” (BH; hypointense on MP2RAGE & hyperintense on FLAIR) ROIs were segmented manually using ITK-SNAP. Based on SWI images lesions were categorized as “rim” (distinct rim shaped iron deposition); “area” (iron accumulation covering the entire lesion); “transition” (transition “area” to “rim” shape); or “no iron” when no iron accumulation was present (Figure 1).
Additionally, all lesions were eroded/dilated 3 times adding/cancelling a voxel defined by the MP2RAGE of the segmented ROI in each step using MINC. After subtraction of the respective dilation/erosion (i.e. 3rd dilation minus 2nd dilation for the outermost layer) this resulted in 7 lesion layer rings that were corrected for intruding gray matter or cerebrospinal fluid using lesion-free masks created in Freesurfer and MINC (Figure 5A).
The mean metabolic ratio values of Ins/tNAA, Ins/tCr, tNAA/tCr and tCho/tCr for each ROI were calculated using MINC.
One-Way ANOVA including Tukey Post-Hoc analysis was performed with a significance threshold of P=0.05.
Results
High quality metabolic maps of mIns/tNAA, mIns/tCr, tNAA/tCr and tCho/tCr were acquired. Significant results were found between “no iron” and “rim” (p<0.01 for mIns/tNAA; p<0.05 for tNAA/tCr) (Figure 3), as well as for “area” (figure 4) and “rim” (p<0.01 for mIns/tNAA; p<0.001 for tNAA/tCr) in mIns/tNAA and tNAA/tCr (Figure 2). Additionally, a significant increase in tNAA/tCr between “no iron” and “area” in tNAA/tCr was found (p<0.05) (Figure 2). There were no significant differences for “transition”, suggesting it is an intermediate state both macroscopically and metabolically. There were no significant differences in tCho/tCr and mIns/tCr, the latter showing consistently high values as known in MS lesions [11](Figure 2). When comparing the different lesion layers (Figure 5), significant differences (i.e p<0.01 between most outer and most inner lesion ring for mIns/tNAA) were present between “no iron” and “iron rim” in mIns/tNAA and tNAA/tCr.Discussion and Conclusion
Increased spatial and spectral resolution at 7T and the full sensitivity of FID-MRSI allowed vizualization of well-demarcated MS lesions and abundant iron deposition, as well as mapping of a neurochemical profile including mIns and tNAA, which are crucial to understand underlying biochemical underpinnings.Previous studies have shown the importance of mIns and tNAA in changes of NAWM in MS [11], and iron accumulation triggering slow expansion of lesions [2].
Our results support these findings and, to the best of our knowledge, attempt for the first time to link metabolic changes to iron deposition, leading to the following remarkable findings:
- overall mIns concentration, which is elevated in “black hole lesions”, is not influenced by iron accumulation
- tNAA is significantly lower in lesions with a pronounced iron rim, supporting their more severe tissue damage [1]
- increased tNAA in the initial stage of diffuse iron accumulation throughout the whole lesion, might represent an intrinsic attempt to enhance neural integrity, supporting the role of iron as a presumed co-regulator of remyelination [12]
- increased tNAA at the lesion borders and steeper tNAA gradient across lesion layers, support hypotheses of slowly expanding lesions with iron rims [2]
Acknowledgements
No acknowledgement found.References
[1] Hametner et al., Brain Pathology 2018; doi: 10.1111/bpa.12643
[2] Dal-Bianco et al., Acta Neuropathologica; doi: 10.1007/s00401-016-1636-z
[3] Absinta et al., JAMA Neurology ;doi:10.1001/jamaneurol.2019.2399
[4] Hangel et al., Neuroimage 2016 [Epub]; doi: 10.1016/j.neuroimage.2016.10.043
[5] Strasser et al., NMR in Biomed 2013; 10.1002/nbm.3019
[6] Heckova et al., Investigative Radiology 2019; doi: 10.1097/RLI.0000000000000531
[7] Saija et al., Neuroimaging Clin N.Am 2009; doi: 10.1016/j.nic.2008.08.002.
[8] Strasser et al., MRM 2017; doi: 10.1002/mrm.26386
[9] Bilgic et al., JMRI 2014; doi: 10.1002/jmri.24365
[10] Povazan et al., Neuroimage 2015; doi: 10.1016/j.neuroimage.2015.07.042
[11] Fernando et al., Brain 2004; doi: 10.1093/brain/awh153
[12] Stephenson et al., Nature Reviews Neurology; doi:10.1038/nrneurol.2014.118
Figures
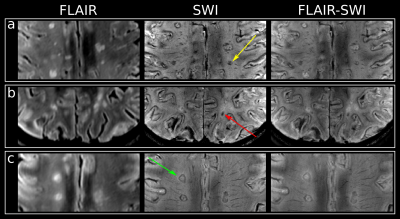
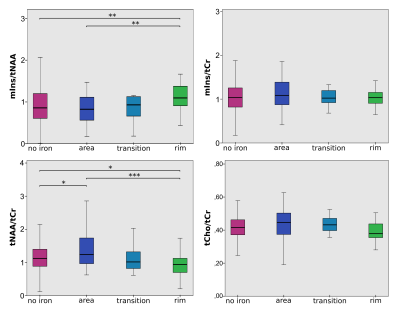
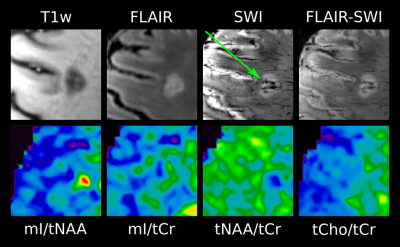
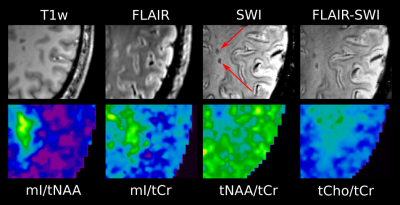
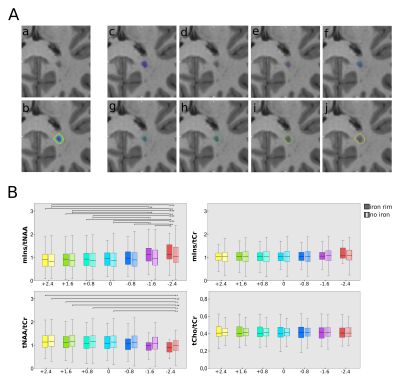
Figure 5:
A: Lesion layers created by expansion/dilation of the lesion: (a) shows the MP2RAGE lesion itself, (b) all lesion rings are overlaid on top of the lesion, (c) represents the originally segmented lesion, and (d)-(j) depict each of the 7 lesion layer rings separately.
B: Boxplot diagram for mIns/tNAA, mIns/tCr, tNAA/tCr and tCho/tCr showing significant differences in metabolite -levels between the lesion layers of “no iron” (striped) and “iron rim” (solid) lesions for tNAA/tCr and mIns/tNAA.