2234
Polarity Dependent Modulation of the Motor Region Using tDCS: A Proton MR Spectroscopy Study1Human Magnetic Resonance Center, Institute for Applied Life Sciences, University of Massachusetts Amherst, Amherst, MA, United States, 2Biomedical Engineering, University of Massachusetts Amherst, Amherst, MA, United States, 3Baystate Medical Center, University of Massachusetts Medical School, Springfield, MA, United States
Synopsis
We studied the effects of motor region tDCS on metabolite levels in a spectroscopic voxel with repeated episodes of 5min of constant current dose levels anticipating stimulation-induced alterations in metabolite concentration at rest. The cumulative change in GABA from before the first tDCS stimulation epoch to after the last epoch showed a significant polarity effect. The first tDCS epoch revealed polarity dependent opposite trends in GABA and Glx from baseline (before stimulation) to post-stimulation. This study provides insights into how tDCS leads to polarity dependent modulation of cerebral metabolites and might provide a model for future MRS stimulation studies.
Introduction
Transcranial direct current stimulation (tDCS) is a neuromodulation procedure that has been frequently applied over the past few years in the therapy of neurological and other diseases1,2. Proton MRS is a non-invasive technique that can measure metabolites' concentrations within a region of interest in the brain. Transcranial direct current stimulation is a non-invasive technique that modulates neurotransmitters, antioxidants, and osmolytes in the brain3-5. MRS studies primarily reviewed the effects of tDCS stimulation over motor regions6-8. There is still a limited understanding of whether and how tDCS modulates brain metabolites with different polarities. In this study, we investigated the effects of motor tDCS (both anode and cathode) on metabolites levels in an MRS voxel with constant dose levels expecting polarity dependent changes in metabolite concentration at rest.Materials and Methods
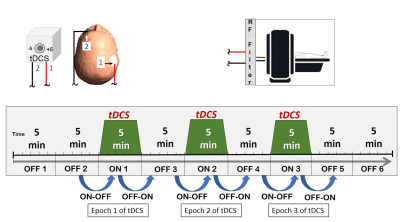
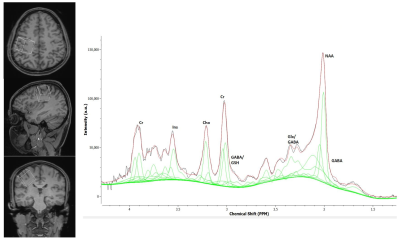
Three healthy volunteers (mean age 39 years) were scanned at a 3T Siemens Skyra system using 32 channel head coil. A PRESS sequence with TR/TE = 200/30 ms, voxel size = 3x3x3 cm3 and 141 averages were used to acquire spectroscopic data to quantify cerebral metabolites. The unsuppressed water reference spectra used to quantify metabolite concentrations. The MRS voxel was placed to cover the right perirolandic region with the motor knob being at its center including both grey and white matter, but carefully avoiding any contribution from the skull or meninges. The entire MRS protocol was about 45 minutes with alternating the tDCS stimulation between OFF and ON in 5 minutes epoches. A T1-weighted 3D MPRAGE sequence with 96 slices of thickness 2.0 mm in sagittal orientation, TR = 1490 ms, TE = 3.36 ms and FOV of 256 × 256 mm were acquired and reconstructed to axial and coronal for the voxel placement. The TARQUIN software package was used to quantify IMA files containing MRS data9. Before the tDCS-MRS scan, all subjects were screened for any implanted electric, metallic, or magnetic material. The skin was inspected before and after the electrode placement. Two rubber pads used as electrodes were placed over the right motor region and the left supraorbital region. Neuroconn DCMC stimulator device was used to generate stimulation signal in the scanner control room. The stimulation signal was transferred from the control room to inside the scanner room through the RF filter panel. Anodal (5 mA) or cathodal (-5 mA) tDCS stimulation was applied concurrently with the third, fifth, and seventh spectroscopy recordings, also called tDCS epochs. Two different analysis schemes were employed to examine the effect of tDCS on cerebral metabolites. In the first scheme, short and cumulative tDCS was evaluated by calculating the difference from metabolites recorded before the first tDCS to after the first tDCS epoch and after the last tDCS epoch, respectively. In the second scheme, the differential effect of tDCS on metabolites either from before stimulation to stimulation (ON-OFF) or from stimulation to post-stimulation (OFF-ON) was evaluated at each epoch. Paired t-tests were performed to assess the effect of polarity on metabolite change as calculated using the two methods described above.Results
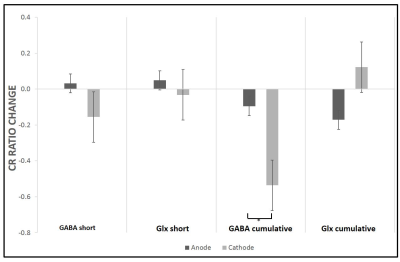
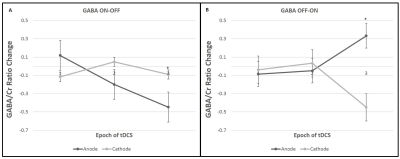
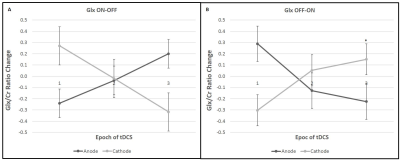
All the metabolites were detected from the perirolandic region. Participants did not have any tolerability issues and significant adverse effects (e.g., severe headaches, seizures, neurological impairments, skin burns) with the high dose tDCS stimulation. Figure 1 shows the schematic diagram of the tDCS setup and 45 minutes of MRS scanning protocol. The MRS voxel location and the spectrum processed by TARQUIN software are shown in Figure 2. The short term and cumulative effect of GABA and Glx (Glutamate+Glutamine) to Creatine changes of Anode and Cathode are shown in Figure 3. Figures 4 and 5 show the differential effect of GABA and Glx to Creatine ratio changes during and after stimulation vs. tDCS epoch. The cumulative effect of tDCS stimulation showed a significant effect of polarity on GABA (p=0.037). The third epoch of 5min tDCS stimulation showed a significant effect of polarity on GABA change compared to its value in the MRS scan acquired immediately before the third epoch (p=0.007). GABA (p=0.048) and Glx (p=0.042) values acquired during and after the third tDCS epoch showed significant polarized changes.Discussion and Conclusions
Differential effects of stimulation in GABA and Glx showed polarity depended trends. The third tDCS epoch showed a significant impact of polarity on change in GABA values (with both before and after stimulation) and change in Glx values from stimulation to post-stimulation recorded with MRS scans. GABA values showed a significant effect of polarity with cumulative tDCS stimulation. Although a change in Glx due to both short and cumulative tDCS and a change in GABA with short tDCS did not show significant effect polarity, these metabolites led polarity dependent trends to either increase or decrease. The current MRS design contributes valuable insight into tDCS effects on the brain. Ongoing work will recruit more subjects to substantiate our findings. Our model of short- and longer-term effects of single and multiple doses of tDCS might provide an interesting framework to examine tDCS-induced cerebral metabolite changes with MRS.Acknowledgements
This research was supported by an NIH BrainInitiative grant (RO1MH111874). Dr. Schlaug also acknowledges support from U01NS102353. We are thankful for and very much appreciate the generous support that Klaus Schellhorn from neuroConn has provided to us with making a state-of-the-art multichannel MR DC stimulator available to us and his helpful suggestions with setting up the concurrent tDCS-MR experiments and being available for any troubleshooting over the years.References
1. Boggio PS, et al. Repeated sessions of non-invasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neuros. 2007;25(2):123–129.
2. Fregni F, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122(1-2):197–209.
3. Bachtiar V, Near J, Johansen-Berg H, Stagg CJ. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife. 2015 Sep 18;4:e08789.
4. Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC‐stimulation‐induced after‐effects of human motor cortex excitability. Brain. 2002 Oct 1;125(10):2238-47.
5. Hunter MA, Coffman BA, Gasparovic C, et al. Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain research. 2015 Jan 12;1594:92-107.
6. Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. J Neurosci. 2009 Apr 22; 29(16):5202-6.
7. Antonenko D, Schubert F, Bohm F, Ittermann B, Aydin S, Hayek D, Grittner U, Flöel A. J Neurosci. 2017 Apr 12; 37(15):4065-4073.
8. Bachtiar V, Johnstone A, Berrington A, Lemke C, Johansen-Berg H, Emir U, Stagg CJ. J Neurosci. 2018 Aug 15; 38(33):7327-7336.
9. Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC. Magn Reson Med. 2011; 65:1–12.
Figures