2231
Protective role of Creatine in chronic hepatic encephalopathy developing brain: in vivo longitudinal 1H and 31P-MRS study1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Animal Imaging and Technology, EPFL, Lausanne, Switzerland, 3Laboratory of Fuctional and Metabolic Imaging, EPFL, Lausanne, Switzerland, 4Service of Clinical Chemistry, University of Lausanne and University Hospital of Lausanne, Lausanne, Switzerland, 5Swiss Center for Liver Disease in Children, University Hospitals Geneva, Geneva, Switzerland
Synopsis
Type-C hepatic encephalopathy (CHE) is a complication of chronic liver disease (CLD). It is known that children are more affected by CLD than adult patients. Bile duct ligated rat (BDL) is a model of CLD-induced CHE. It was shown that rats having acquired CLD as pups display more profound neurometabolic disturbances than adults. Cr-treatment showed a positive effect in youngP21 BDL-rats resulting in less pronounced metabolic changes. Our aim was to test whether Cr-supplementation dampens the changes observed in CHE in a longitudinal model of CLD acquired in early childhood-P15 and if these changes are similar to those in P21-rats.
Introduction
Type-C hepatic encephalopathy (CHE) is a complication of chronic liver disease (CLD), difficult to diagnose in its early stages. It is known that children are more affected by CLD and its related toxic accumulation of ammonium (NH4+) and glutamine (Gln) than adult patients, with long-lasting cognitive deficits after liver transplantation1–4.The bile duct ligated rat (BDL) is a model of CLD-induced CHE validated in the adult and developing brain5,6. It has been shown that rats having acquired CLD as pups display more profound neurometabolic disturbances than adults with the same disease (more important Gln increase, stronger osmotic and antioxidant response, stronger decrease of total-creatine(tCr))6. A decrease in tCr has been shown in developing rat brain-cells aggregates after NH4+ exposure, due to downregulation of Cr synthesis7. As such, exploring methods to efficiently sustain Cr concentration in CHE may have potentially far-reaching clinical implications.
Recently differences in brain metabolic changes between post-natal 15(P15) and post-natal 21(P21) operated BDL-rats have been shown, suggesting that age of disease onset and its coincidence with neurodevelopmental processes play an important role and may result in different vulnerability to the disease8. Moreover, NH4+-induced impaired axonal growth was shown to be rescued by Cr-treatment in organotypic 3D brain cell cultures9 and P21 BDL-rats treated with Cr showed less pronounced metabolic changes10.
Therefore, we hypothesized that high Cr diet might be beneficial in CHE in P15 BDL-rats. Our aim was to test whether oral Cr supplementation dampens the neurometabolic changes observed in CHE in a longitudinal model of CLD acquired in childhood and if these changes are similar to those in P21 rats.
Methods
BDL surgery was performed on 6 male Wistar rats at P15. BDL rats were compared with sham(n=6) operated animals at the same age to consider the ongoing brain development and were divided into 4 groups: BDL(n=3), BDL+Cr(n=3), sham(n=3), sham+Cr(n=3). Rats from treated groups received high Cr supplemented diet with a concentration of 40g/kg.1H-MRS and 31P-MRS and blood tests were preformed longitudinally every two weeks(week2-4-6).
MRS experiments were done using a 9.4T-system(Varian/Magnex Scientific) together with home-built coil (quadrature 1H-loops with a single 31P-loop). 1H-MRS spectra were acquired in hippocampus (2x2.8x2mm3) using the SPECIAL sequence (TE=2.8ms)11. First and second order shims were adjusted using FASTMAP12. 31P-MRS spectra were acquired using a non-selective AHP pulse for excitation, localized by OVS(x,z) and 1D-ISIS(y) (TR=8s, 384avg.), WALTZ-16 for NOE and 1H-decoupling in VOI=5x9x9mm3. 31P-MR spectra were quantified using AMARES(jMRUI)13 and normalized for each rat using its PCr concentration from 1H-MRS acquired in VOI=4x7.5x6.5mm3 centered in 31P-VOI.
Results and discussion
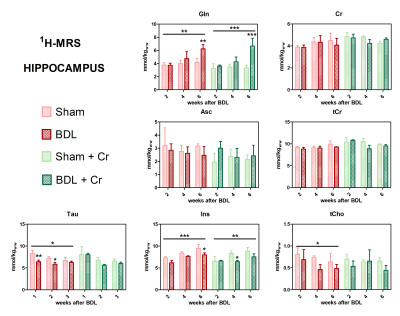
All BDL-operated rats showed increase in plasma-bilirubin and blood-NH4+ validating the presence of CLD. Some improvements in the neurometabolic profile were noticed for BDL rats under Cr-treatment when looking at the differences Sham/BDL vs Sham+Cr/BDL+Cr. Cr-treatment seemed to restore the decrease in Cr and tCr causing a higher Cr in BDL+Cr(+13% at week6). Of note, all treated rats seem to show higher Cr concentrations in the brain following treatment, probably due to the more permeable and immature BBB at this age. Decrease in Asc concentration is a recently shown hallmark in HE (here not yet significant due to small n) and as previously shown8 appeared later in P15-BDL rats(week6) compared to P21-BDL rats(week4). Cr-treatment restored Asc in BDL rats emphasizing the antioxidant role of Cr14. Treatment seems to have a positive effect on other osmolytes(Ins,Tau and tCho) which appear to have a less significant decrease in BDL+Cr than in BDL even though Gln is as high in treated rats as in non-treated (Fig.1).This study confirmed a previously shown delayed increase in glutamine(Gln), as a consequence of NH4+ detoxification, for P15-BDL rats probably caused by immature glutamine synthetase15 enzyme at a very early age delaying the NH4+ detoxification. There was no effect on Gln due to Cr-treatment for the P15-BDL rats(Fig.1), although a positive impact of Cr-treatment was seen only at week8 in the P21 rats10. Because of high impact of NH4+ on an immature brain, P15-BDL rats didn’t survive so late in the disease.
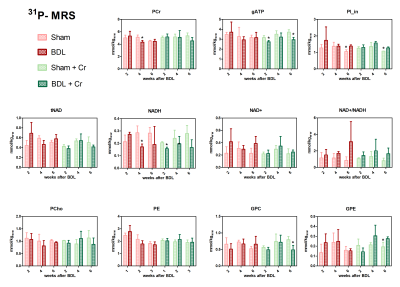
Treated BDL rats showed a stable PCr concentration at week4(ns, BDL+Cr-vs-Sham+Cr) compared to BDL without treatment which displayed a significant decrease (*p<0.05, BDL-vs-Sham), with this effect diminishing at week6. Treatment had no effect on ATP, contrary to P21 results which showed a positive effect of Cr for both PCr and ATP at week610. However, treated BDL rats have a more stable tNAD pool (similar to sham/sham+Cr) compared to non-treated ones. Higher variations in NADH and NAD+/NADH ratios indicate a more unstable redox state for non-treated BDL rats indicating an increased oxidative stress (Fig.3).
Conclusion
Our preliminary results showed an improved neurometabolic profile due to Cr supplementation emphasizing the antioxidant role of Cr. We know that there is a different vulnerability to the disease depending on age in CHE and Cr-supplementation seemed to confirm this vulnerability by a different response between P15 and P21-BDL rats (Gln, PCr, ATP). The positive effect on Asc and other osmolytes point towards the need of combinatorial treatments in CHE. Additional studies are required to confirm our results and to investigate if these differences in neurometabolism due to Cr-supplementation translate also into different neurological outcome.Acknowledgements
We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole polytechnique fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG).References
1. Ng V, Nicholas D, Dhawan A, et al. Development and validation of the pediatric liver transplantation quality of life: A disease-specific quality of life measure for pediatric liver transplant recipients. J Pediatr. 2014;165:547–555. doi:10.1016/j.jpeds.2014.05.024
2. Caudle SE, Katzenstein JM, Karpen S, McLin V. Developmental assessment of infants with biliary atresia: differences between boys and girls. J Pediatr Gastroenterol Nutr. 2012;55(4):384-389. doi:10.1097/MPG.0b013e318259ed20
3. Caudle SE, Katzenstein JM, Karpen SJ, McLin VA. Language and Motor Skills Are Impaired in Infants with Biliary Atresia Before Transplantation. J Pediatr. 2010;156(6). doi:10.1016/j.jpeds.2009.12.014 4. Cagnon L, Braissant O. Hyperammonemia-induced toxicity for the developing central nervous system. Brain Res Rev. 2007;56(1):183-197. doi:10.1016/j.brainresrev.2007.06.026
5. Braissant O, Rackayová V, Pierzchala K, Grosse J, McLin VA, Cudalbu C. Longitudinal neurometabolic changes in the hippocampus of a rat model of chronic hepatic encephalopathy. J Hepatol. 2019;71(3):505-515. doi:10.1016/j.jhep.2019.05.022
6. Rackayova V, Braissant O, Rougemont A-L, et al. Longitudinal osmotic and neurometabolic changes in young rats with chronic cholestatic liver disease. Sci Rep. 2020;10(1):7536. doi:10.1038/s41598-020-64416-3
7. Braissant O, Cagnon L, Monnet-Tschudi F, et al. Ammonium alters creatine transport and synthesis in a 3D culture of developing brain cells, resulting in secondary cerebral creatine deficiency. Eur J Neurosci. 2008;27(August 2007):1673-1685. doi:10.1111/j.1460-9568.2008.06126.x
8. Rackayova V, Braissant O, Mclin V, Cudalbu C. Chronic hepatic encephalopathy in early developing brain , neurometabolic changes differ depending on the age of disease onset , in vivo longitudinal 1H MRS study : 2018;(ISMRM 2018):4-7.
9. Braissant O, Henry H, Villard A-M, et al. Ammonium-induced impairment of axonal growth is prevented through glial creatine. J Neurosci. 2002;22(22):9810-9820.
10. Rackayova V, Braissant O, Sessa D, et al. Protective effect of high creatine diet during chronic hepatic encephalopathy in young rats , an in vivo longitudinal 1H and 31P MRS study. 2017;4(ISMRM 2017):9-12.
11. Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006;56(5):965-970. doi:10.1002/mrm.21043
12. Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319-323. doi:10.1002/(SICI)1522-2594(200002)43:2<319::AID-MRM22>3.0.CO;2-1
13. Vanhamme L, Van Huffel S. AMARES: Advanced Method for Accurate, Robust and Efficient Spectral fitting of MRS data with use of prior knowledge. 1997;43(129):1-2. http://www.esat.kuleuven.be/sista/yearreport96/node2.html
14. Sestili P, Martinelli C, Colombo E, et al. Creatine as an antioxidant. Amino Acids. 2011;40(5):1385-1396. doi:10.1007/s00726-011-0875-5
15. Bayer SM, McMurray WC. The Metabolism of Amino Acids in Developing Rat Brain. J Neurochem. 1967;14:695-706.
Figures