2225
Exploring Brain Metabolites in Pediatric Concussion: An A-CAP Study1Radiology, University of Calgary, Calgary, AB, Canada, 2Pediatrics, University of Alberta and Stollery Children's Hospital, Edmonton, AB, Canada, 3Pediatrics, University of British Columbia, Vancouver, BC, Canada, 4Psychology, University of Montreal and St Justine Hospital, Montreal, QC, Canada, 5Pediatrics and Emergency Medicine, University of Ottawa and Children's Hospital of Eastern Ontario, Ottawa, ON, Canada, 6Alberta Children's Hospital, Calgary, AB, Canada, 7Psychology, University of Calgary, Calgary, AB, Canada
Synopsis
Disruptions in brain metabolites following concussion are commonly reported in the literature. These studies often have different timings following injury, are limited to adults and have limited sample size. Using magnetic resonance spectroscopy (MRS), we show in the largest MRS in pediatric concussion study to date, that there are no group differences in metabolites between concussion and orthopedic injury at acute injury. Glx was associated with cognitive symptoms but no other metabolites showed a relationship with symptoms. No difference between groups may indicate that metabolic disturbance following injury may be a broad indicator of injury or alterations are regionally specific.
Introduction
One out of five children will sustain a concussion before the age of 18 (1). Although research in concussion is increasing, there is still no prognosticative biomarker to indicate post-injury outcomes. Magnetic resonance spectroscopy (MRS) was used to explore several key metabolites that may serve as biomarkers. Previous studies suggest tNAA (NAA+NAAG, a marker of neuronal integrity) decreases (2–4), and Glx (Glu+Gln, a marker of excitatory tone), tCho (GPC+Cho+PCho, indicates membrane turnover), and tCr (Cr+PCr, indicates bioenergetics) increase following concussion (4–9). Prior work has primarily studied adults with concussion and has been limited by small sample sizes. In the largest MRS-concussion dataset acquired to date, we examined brain metabolites in pediatric concussion and their relationship to symptoms.Methods
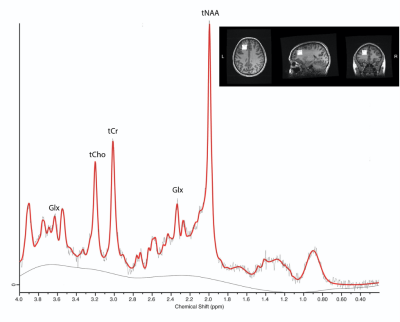
Children (age 8-16.99 y) with concussion and orthopedic injury (OI control group owing to their shared exposure to a traumatic injury) were recruited across two emergency departments as part of the advancing concussion assessment in pediatrics study (10). Assessments were conducted within 10 days of injury and included a research 3T Magnetic Resonance Imaging (GE 750w, and GE 750) scan including proton MRS. MRS data was collected using a short-echo single voxel PRESS (TE=30ms, TR=2s, 8ml voxel, 96 averages, figure 1) from the left dorsolateral prefrontal cortex, as this region is functionally relevant to concussion symptoms of impaired executive function and working memory.MRS data was preprocessed in FID-A (11), quantified in LCModel (12), and subsequently tissue corrected (13). An example spectrum is shown in figure 1. Data quality was assessed by visual inspection and thresholds for quality were a signal-to-noise (SNR) ratio of at least 45, and a Cramer-Rao lower bound of less than 20%.
The Health and Behaviour Inventory (HBI) questionnaire was used to quantify concussion symptoms, which includes cognitive and somatic scores from both parent and child reports (14).
ANCOVAs were used to study differences in metabolites between concussion and OI groups while controlling for age, sex, and site. Multivariate regressions including all four metabolites was used to determine the relationship between metabolites for each of the four HBI concussion symptom scores (i.e. a model for parent somatic, parent cognitive, child somatic, child cognitive) while controlling for age, sex, and site.
Results
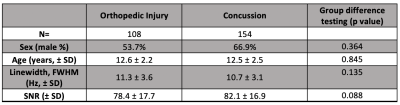
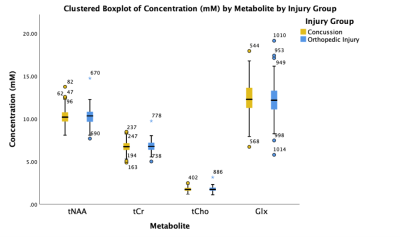
Data was acquired from 262 participants: 154 with concussion and 108 OI. Demographic and MRS data quality were comparable between groups (Table 1).There were no significant differences in tNAA, tCr, tCho, and Glx between the concussion and OI groups (p>0.05, figure 2).
The regression model for metabolite effects on HBI parent report, was only significant in predicting the somatic concussion symptoms (p=0.0001, F=4.966); however, the only significant independent predictors in the model were age and sex (p=0.0001, p=0.038, respectively).
The regression models for the effect of metabolites on the child report HBI were significant for both cognitive (F=3.216, p=0.003) and somatic symptoms (F=2.635, p=0.014) following concussion. For cognitive symptoms, the significant predictors were Glx and age (p=0.044, p=0.013) and for somatic symptoms, sex was a significant predictor (p=0.04).
Discussion
We found no differences in metabolites (tNAA, tCr, tCho and Glx) between pediatric concussion and OI in the sub-acute period. This may indicate that cortical metabolic differences is associated with general injury rather than concussion specifically. Alternatively, metabolite differences may be subtle in pediatrics or only seen in subgroups that are not identified in this study.Our results are in contrast to previous literature that has consistently shown tNAA decreases following concussion (2–4), however, these studies have had white matter voxels. A recent study suggests decreases in tNAA are driven by decreases in NAAG (15). As grey matter has less NAAG than white matter, the mixed voxel tissue-content in the current study may have limited the ability to detect change in tNAA. Similarly, studies documenting the change in tCr and tCho have been in white matter regions (5,8,16). Glx may be differentially affected by tissue as literature suggests Glx increased in white matter (5,17) and decreased in grey matter (5,8) with concussion.
While tissue correction was performed, future analysis will examine the effects of grey and white matter. The smaller groups in previous literature may have been more homogeneous samples, or the adult brain shows different metabolic responses compared to a developing brain. Furthermore, it is difficult to compare data at differing time points post-injury.
Of the four multivariate models examining the relationship between metabolites and symptoms, the models for parental and child reports of somatic symptoms, and the child report of cognitive symptoms were significant. Glx was a significant predictor for child report cognitive symptoms. As Glx was observed to decrease in response to increasing symptoms, this could indicate in the sub-acute phase of recovery, the pediatric brain compensates for hyperexcitability by decreasing glutamatergic metabolites. The other models (child and parent reports of somatic symptoms) were driven by age and/or sex. Further investigation of the age-metabolite relationship is necessary to fully understand this finding as metabolite levels are known to change with development.
Significance
This is the largest MRS dataset in the sub-acute phase of pediatric concussion. No group metabolite differences were found between injury groups and only Glx had significantly predicted cognitive symptoms.Acknowledgements
Funding for this study was provided by: The Canadian Institutes of Health Research, Alberta Children’s Hospital Research Institute and an Alberta Graduate Excellence Scholarship.References
1. MacMaster FP, McLellan Q, Harris AD, et al. N -Acetyl-Aspartate in the Dorsolateral Prefrontal Cortex Long after Concussion in Youth. J Head Trauma Rehabil. 2019; doi: 10.1097/HTR.0000000000000535.
2. Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200.
3. Veeramuthu V, Seow P, Narayanan V, et al. Neurometabolites Alteration in the Acute Phase of Mild Traumatic Brain Injury (mTBI): An in Vivo Proton Magnetic Resonance Spectroscopy (1H-MRS) Study. Acad Radiol. Elsevier Inc.; 2018;25(9):1167–1177. doi: 10.1016/j.acra.2018.01.005.
4. Johnson B, Zhang K, Gay M, et al. Metabolic alterations in corpus callosum may compromise brain functional connectivity in MTBI patients: An 1H-MRS study. Neurosci Lett. Elsevier Ireland Ltd; 2012;509(1):5–8. doi: 10.1016/j.neulet.2011.11.013.
5. Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, Mayer AR. A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. J Neurotrauma. 2011;28(1):1–11. doi: 10.1089/neu.2010.1578.
6. Kierans AS, Kirov II, Gonen O, et al. Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology. 2014;82(6):521–528. doi: 10.1212/WNL.0000000000000105.
7. Shutter L, Tong KA, Holshouser BA. Proton MRS in acute traumatic brain injury: Role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004;21(12):1693–1705. doi: 10.1089/neu.2004.21.1693.
8. Gasparovic C, Yeo R, Mannell M, et al. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: An 1H-magnetic resonance spectroscopy study. J Neurotrauma. 2009;26(10):1635–1643. doi: 10.1089/neu.2009.0896.
9. Lin AP, Liao HJ, Merugumala SK, Prabhu SP, Meehan WP, Ross BD. Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):208–223. doi: 10.1007/s11682-012-9181-4.
10. Yeates KO, Beauchamp M, Craig W, et al. Advancing Concussion Assessment in Pediatrics (A-CAP): A prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: Protocol study. BMJ Open. 2017;7(7):1–14. doi: 10.1136/bmjopen-2017-017012.
11. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)—An open source, MATLAB-based toolkit. Magn Reson Med. 2017;77(1):23–33. doi: 10.1002/mrm.26091.
12. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698.
13. Near J, Harris AD, Juchem C, et al. Preprocessing, analysis and quantification in single‐voxel magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. 2020;(December 2019):1–23. doi: 10.1002/nbm.4257.
14. McCauley SR, Wilde EA, Anderson VA, et al. Recommendations for the Use of Common Outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012;29(4):678–705. doi: 10.1089/neu.2011.1838.
15. Menshchikov P, Ivantsova A, Manzhurtsev A, et al. Separate N-acetyl aspartyl glutamate, N-acetyl aspartate, aspartate, and glutamate quantification after pediatric mild traumatic brain injury in the acute phase. Magn Reson Med. 2020;84(6):2918–2931. doi: 10.1002/mrm.28332.
16. Sarmento E, Moreira P, Brito C, Souza J, Jevoux C, Bigal M. Proton spectroscopy in patients with post-traumatic headache attributed to mild head injury. Headache. 2009;49(9):1345–1352. doi: 10.1111/j.1526-4610.2009.01494.x.
17. Schranz AL, Manning KY, Dekaban GA, et al. Reduced brain glutamine in female varsity rugby athletes after concussion and in non-concussed athletes after a season of play. Hum Brain Mapp. 2018;39(4):1489–1499. doi: 10.1002/hbm.23919.
Figures