2223
1H MRS of the Pediatric Brain Metabolism During Cardiopulmonary Bypass (CPB) Surgery1Radiology, Stanford University, Stanford, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States, 3Cardiothoracic Surgery, Stanford University, Stanford, CA, United States
Synopsis
We used single-voxel 1H MRS at 3T to study acute brain metabolic changes during cardiopulmonary bypass (CPB) surgery in a neonatal piglet model, with the overall goal of determining optimal surgical parameters for minimizing brain injury. The recent improvements in proton spectroscopy and MR scanner performance allowed robust measurement of dynamic brain water and metabolic changes during hypothermia and circulatory arrest. Key findings include a linear relationship between unsuppressed water and brain temperature, observation of the theoretically predicted NMR “powder pattern” from intravascular water during hypoxia, and quantitative measurements of glucose, lactate, and phosphocreatine metabolic rates vs temperature.
Introduction
Congenital heart disease affects 1% of births per year in the US. Approximately 25% of these babies require surgery during their first year of life, with more than 50% of more critical patients suffering from some form of neurological impairment or developmental delay. We used an optimized 3T single-voxel 1H MRS to study acute brain metabolic changes during deep hypothermic circulatory arrest (DHCA) cardiopulmonary bypass (CPB) surgery in a neonatal piglet model [1], with the overall goal of determining optimal surgical parameters for minimizing brain injury.Methods
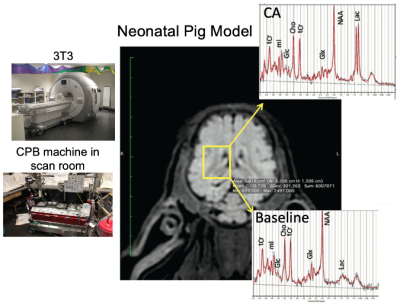
Piglets (N=6, newly weaned, 2 wks old, 10.3±0.9 kg) were put on a cardiopulmonary bypass pump and placed in a GE MR750 scanner (GE Healthcare, Waukesha, WI) equipped with an 8-channel array “knee” coil. Localizer T1 FSE images were acquired, and a 12 x 12 x 15 mm3 right midbrain voxel was prescribed using a TE/TR=30/2000 ms sLASER pulse sequence (see Figure 1). Dynamic spectra of 128 or 256 averages were acquired with a 5000 Hz bandwidth were the acquired over 2-3 hours covered baseline, cooling, circulatory arrest, and recovery periods. The physiological range for these studies included hypothermia temperatures of 19.1±1.9°C (N=5) and 37°C (N=1) with initial blood glucose levels also manipulated. Preprocessing of the resulting spectral included pure water subtraction to achieve a baseline neutral residual water less than 5x metabolite level. All spectra were then fit using LCModel software [2] using experimentally measured basis functions (acquired at physiological pH, 37ºC, and 18ºC) for each of 23 metabolites. Metabolites were quantified based on an assumed brain total creatine (tCr = creatine + phosphocreatine, PCr) of 8 mM.Results
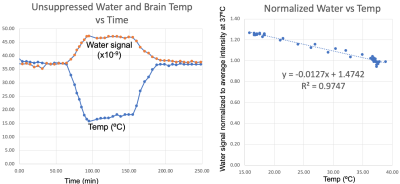
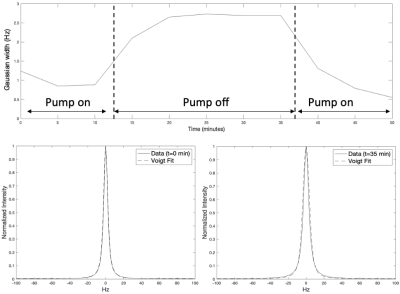
The results from this study are summarized in Figure 2-5 with specific findings regarding information available from the unsuppressed water signal, suppressed water signal, and energy metabolites. A robust linear relationship (R2= 0.97 ± 0.02, see Figure 2), was found between the unsuppressed water signal (peak integral) and temperature with the findings validated using the chemical shift between water and NAA as a standard [3]. This finding is surprising, given that MR signal intensity versus temperature is a complicated function of T1-dependent tissue types, polarization, and perfusion/diffusion effects [4]. Given the high SNR of the unsuppressed water signal, this finding could have implications for MRI temperate mapping applications.With the onset of hypoxia, the unsuppressed water peak broadens, likely due to the build-up of deoxyhemoglobin, and Figure 3 demonstrates that the broadening peak is very well modeled using a Voigt lineshape (exponential T2 decay convolved with Gaussian broadening). CBP pig studies with and without hypothermia demonstrated that this hypoxia-dependent water peak broadening was independent of brain temperature.
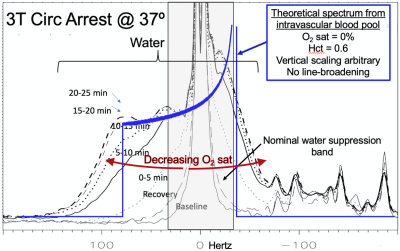
In contrast to the unsuppressed water, strongly asymmetric broadening of the residual suppressed water signal was observed with hypoxia. Given a fixed water suppression bandwidth of ~75 Hz, this asymmetric broadening likely results from spatial regions with B0 inhomogeneity significantly shifted from the primary water peak. Based on modeling vessels and capillaries as cylinders randomly distributed in space, signal from deoxygenation intravascular water has been theoretically predicted to exhibit a classic NMR “powder pattern” by Spinger, et al. [5]. We hypothesize that under conditions of extreme hypoxia (in this case 25 min post circulatory arrest at 37ºC) intravascular water, despite composing only 2-3% of brain water, is frequency-shifted by the presence of deoxyhemoglobin to the extent that the corresponding signal is detectable in the residual water from a single-voxel 1H MRS study. Figure 4 shows the broadening asymmetric residual water signal observed from dynamically acquired brain spectra with falling O2-saturation. The theoretical power pattern plot of fully deoxygenated intravascular water is overlayed on the acquired data.
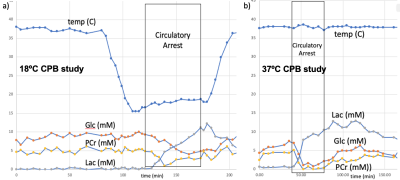
With respect to the brain metabolites, as shown by a representative study presented in Figure 5, in addition to the large lactate build-up expected with circulatory arrest, we were also able to obtain robust measurement of changes in glucose and phosphocreatine. The average metabolic rate of lactate production during hypothermic circulatory arrest (0.20±0.04 μmoles/g/min) was approximately twice the rate of glucose consumption (0.11±0.04 μmoles/g/min), indicated the predominance of anaerobic metabolism. The additional phosphocreatine consumption (0.04±0.02 μmoles/g/min) measurements allowed estimation of an average anaerobic ATP production rate of 0.26±0.02 μmoles/g/min. These rates were approximately 5x higher at 37ºC.
Discussion
This study used 3T single-voxel 1H MRS in a neonatal piglet model with the ultimate goal of determining optimal surgical parameters for minimizing brain injury. Findings include robust measures of brain temperature, hypoxia, and energy metabolism and demonstrate the large information content available from in vivo 1H MRS. To our knowledge these are the first in vivo reports of the observation of a power pattern from deoxygenated vessels and the demonstration of PCr changes with 1H rather than less sensitive 31P spectroscopy. Future analysis will include assessment of temporal patterns in additional metabolites (including glutamate, glutamine, glutathione, and ascorbate), measurement of metabolite T1 and T2 changes with hypothermia and hypoxia, and correlation with post-surgical measurements of learning deficits in recovered animals.Acknowledgements
NIH grants P41EB015891 and R01MH110683References
[1] Hanley, F. L., Ito, H., Gu, M., Hurd, R., Riemer, R. K., and Spielman, D. (2020) Comparison of dynamic brain metabolism during antegrade cerebral perfusion versus deep hypothermic circulatory arrest using proton magnetic resonance spectroscopy. J Thorac Cardiovasc Surg 160, e225-e227
[2] Provencher, S. W. (2001) Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomedicine, 14(4), 260–264. https://doi.org/10.1002/nbm.698
[3] Corbett, R. J., Laptook, A. R., Tollefsbol, G., and Kim, B. (1995) Validation of a noninvasive method to measure brain temperature in vivo using 1H NMR spectroscopy. J Neurochem 64, 1224-1230.
[4] Rieke, V and Butts Pauly, K, (2008) MR Thermometry, J Magn Reson Imaging, 27(2): 376-390.
[5] Springer, C.S., Patlack, C.S., Palyka, I, and Huang W., (1999) Principles of Susceptibility Contrast-Based Functional MRI: The Sign of the Functional MRI Response, Functional MRI, eds Moonen, C.T.W. and Bandettini, P.A., Springer-Verlag, Berlin, Chapter 9, pp. 91-102.
Figures