2217
Examining the relationship between glutathione and post-traumatic headache in patients with persistent post-concussive symptoms
Julie M Joyce1,2,3,4, Leah J Mercier2,4,5, Parker L La1,2,3,4, Tiffany Bell1,2,3, Julia M Batycky2,4,5, Chantel T Debert2,3,4,5, and Ashley D Harris1,2,3,4
1Department of Radiology, University of Calgary, Calgary, AB, Canada, 2Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 3Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada, 4Integrated Concussion Research Program, University of Calgary, Calgary, AB, Canada, 5Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
1Department of Radiology, University of Calgary, Calgary, AB, Canada, 2Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 3Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada, 4Integrated Concussion Research Program, University of Calgary, Calgary, AB, Canada, 5Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
Synopsis
Persistent post-concussive symptoms (PPCS) are debilitating symptoms that endure beyond the usual recovery period after concussion. Post-traumatic headache is one of the most common symptoms of concussion and its pathophysiology remains poorly characterized. Oxidative stress may contribute to the symptoms seen in PPCS patients. Here, we use edited magnetic resonance spectroscopy to investigate levels of the antioxidant glutathione in the anterior cingulate and sensorimotor cortices in adults with PPCS relative to controls. In the anterior cingulate—a critical hub in the default mode network—we found a significant positive correlation between glutathione and functional impact of headache in PPCS patients.
INTRODUCTION
Headache is one of the most common symptoms of concussion with an estimated 47-95% of concussion patients developing chronic post-traumatic headache.1,2 While some concussion patients experience intermittent headache, others are burdened with constant headache.3 The pathophysiology of post-traumatic headache is unclear. In the absence of a biological target, current treatment options are limited to symptom management.Oxidative stress contributes to secondary cellular injury after concussion and may play a role in the perpetuation of persistent post-concussive symptoms (PPCS).4 This makes antioxidants, such as glutathione (GSH), desirable targets for concussion research.
GSH can be measured non-invasively using magnetic resonance spectroscopy (MRS). However, GSH is challenging to measure using conventional MRS methods as it has multiplet signals due to scalar coupling and is obscured by larger signal peaks.5 J-difference spectral editing techniques simplify the spectrum, enabling quantification of GSH.6
This project uses advanced spectral editing methods to examine GSH in patients with PPCS compared to controls. We will also present initial findings of an ongoing study examining GSH response to an aerobic exercise treatment intervention for PPCS.
METHODS
Thirty-three participants (7M/26F) aged 23-61 years (16 PPCS; 17 age- and sex-matched controls without history of concussion) completed an MRS scan at 3T (MR750w, General Electric).All participants with PPCS completed symptom questionnaires including the Rivermead Post-Concussion Symptom Questionnaire (RPQ)7, Post-Concussion Syndrome Checklist (PCSC)8 and Headache Impact Test (HIT-6)9. The Post-Concussion Syndrome Checklist was used to identify participants with constant headache (headache duration score of 5) or intermittent headache (headache duration score of 3-4).
Following the baseline MRS scan, participants with PPCS were randomized to a six-week aerobic exercise or stretching group. Thirteen participants with PPCS completed a follow-up MRS scan and questionnaires.
Each imaging session began with the acquisition of a T1-weighted anatomical image for voxel placement and tissue segmentation (BRAVO; TR/TE = 7.30/2.66 ms, 1 mm3 isotropic voxels). 3x3x3 cm3 voxels were placed in the anterior cingulate and right sensorimotor cortices.
GSH was measured using a Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy (HERMES) spectral editing scheme: TR/TE = 2000/80 ms, editing pulses at 4.56 and 1.9 ppm, 320 averages. HERMES data were analyzed using Gannet3.1.10 While HERMES enables simultaneous acquisition of GABA and GSH data, GABA was not part of our oxidative stress hypothesis and is not examined here.
Independent samples t-tests compared GSH levels and questionnaire scores between groups and brain regions. The relationship between GSH levels and symptom scores in PPCS participants was assessed using Pearson’s correlations.
RESULTS
No significant differences in GSH concentration were found between patients with PPCS and controls in either brain region. However, PPCS participants with constant headache (n = 10) were found to have significantly higher anterior cingulate cortex GSH than controls, t(21) = 2.47, p = 0.022 (Figure 2).Pearson’s correlation revealed a significant association between baseline anterior cingulate GSH and HIT-6 scores with higher HIT-6 scores indicative of greater functional impact of headache, r(16) = 0.507, p = 0.045 (Figure 3).
GSH levels varied by brain region. Higher levels of GSH were found in the anterior cingulate relative to the right sensorimotor cortex in both patients with PPCS (t(30) = 13.96, p <.001) and controls (t(26) = 13.36, p <.001) (Figure 4).
Preliminary analyses of PPCS participants who completed the aerobic exercise intervention revealed a non-significant trend towards a reduction in anterior cingulate cortex GSH between baseline and follow-up (Figure 5). Participants who completed the aerobic exercise intervention reported lower total HIT-6 scores reflecting reduced functional impact of headache, but not total RPQ or PCSC scores, at the follow-up visit.
DISCUSSION
Here we show that, compared to age- and sex-matched controls without history of concussion, PPCS patients with constant headache had higher GSH in the anterior cingulate cortex. Moreover, higher GSH in the anterior cingulate was associated with greater functional impact of headache in PPCS patients. This appears to be a regionally specific finding as GSH in the sensorimotor cortex showed no relationship with headache.GSH protects cells from oxidative stress. Exposure to chronic oxidative stress may trigger an upregulation of antioxidant defenses, which has been supported by ex vivo biochemical assays.5 Enhanced GSH synthesis may be a compensatory mechanism to respond to ongoing oxidative stress in the brains of patients with PPCS. However, many PPCS patients are on medications for symptom management which may also influence GSH levels and needs further exploration.
Notably, the group differences in GSH and the relationship between GSH and symptoms were region-specific. Higher GSH in the anterior cingulate cortex may reflect a regional susceptibility to oxidative stress.
Our preliminary exercise intervention findings show a trend towards a reduction in GSH concentration accompanied with a decrease in functional impact of headache scores in PPCS participants who completed the six-week aerobic exercise intervention. These changes suggest a reduction in oxidative stress reflecting a healthier brain state.
CONCLUSION
We conclude that GSH concentrations are increased in PPCS patients with high functional impact of headache and that these effects are brain region-specific.Acknowledgements
Research was supported by the New Frontiers in Research Fund, Foundations of Physical Medicine and Rehabilitation Grant, Hotchkiss Brain Institute PFUND Grant and Alberta Graduate Excellence Scholarship.References
- Defrin R. Chronic post-traumatic headache: clinical findings and possible mechanisms. J Man Manip Ther. 2014;22(1):36-44.
- Ashina H, et al. Post-traumatic headache: epidemiology and pathophysiological insights. Nat Rev Neurol. 2019; 15(10):607-617.
- Dwyer B and N Zasler. Post-traumatic cephalalgia. NeuroRehabilitation. 2020; 47(3): 327-342.
- Giza CC and DA Hovda. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75 Suppl 4: S24-S33.
- Choi IY, et al. Spectral editing in 1H magnetic resonance spectroscopy: Experts' consensus recommendations. NMR Biomed. 2020; e4411.
- Saleh MG, et al. Simultaneous edited MRS of GABA and glutathione. Neuroimage. 2016; 142:576-582.
- King NS, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587-592.
- Gouvier WD, et al. Postconcussion symptoms and daily stress in normal and head-injured college populations. Arch Clin Neuropsych. 1992;7:193-211.
- Kosinski M, et al. A six-item short-form survey for measuring headache impact: The HIT-6. Qual Life Res. 2003;12(8):963-974.
- Edden RA, et al. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445-1452.
Figures
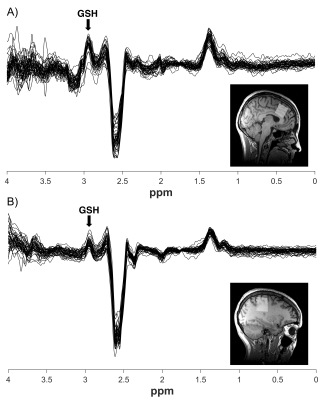
Figure 1. Example voxel placement and overlay of all HERMES spectra for A) Anterior cingulate cortex (mean linewidth = 13.75 ± 2.69 Hz) and B) Right sensorimotor cortex (mean linewidth = 9.65 ± 3.01 Hz). Seven spectra of poor quality (e.g., had unresolvable subtraction artifact, poor model fit and/or >20 Hz frequency drift) were excluded from analyses.
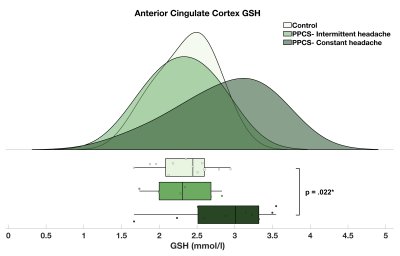
Figure 2. Raincloud plot depicting the GSH concentration distribution (mmol/l) in the anterior cingulate cortex of PPCS participants with intermittent (mean = 2.31 ± 0.41; n = 6) and constant headache (mean = 2.86 ± 0.61; n = 10) and matched, injury-free controls (mean = 2.36 ± 0.37; n = 13).
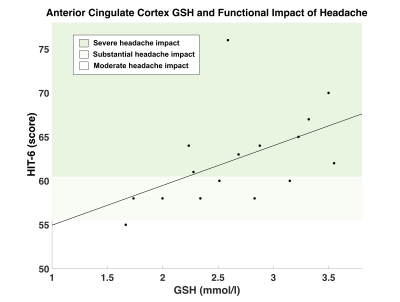
Figure 3. Association between functional impact of headache and GSH concentration (mmol/l) in the anterior cingulate cortex. GSH in the anterior cingulate cortex was positively correlated with HIT-6 scores; (higher HIT-6 scores indicates greater functional impact of headache) r(16) = 0.507, p = 0.045*. HIT-6 score classifications: (1) little-to-no impact (score: 36-39), (2) moderate impact (score: 50-55), (3) substantial impact (score: 56-59), (4) severe impact (score 60-78).
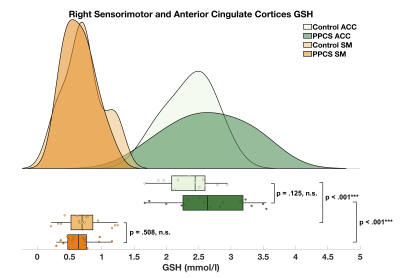
Figure 4. Raincloud plot depicting the GSH concentration distribution (mmol/l) in the right sensorimotor (PPCS mean = 0.65 ± 0.24; Control mean = 0.72 ± 0.31) and anterior cingulate cortices (PPCS mean = 2.66 ± 0.59; Control mean = 2.36 ± 0.37) of PPCS participants and age- and sex-matched controls.
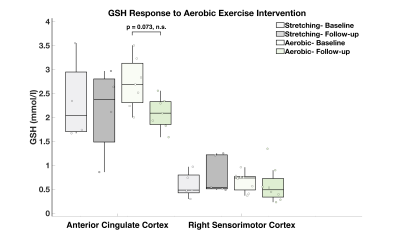
Figure 5. Group comparisons of GSH concentrations (mmol/l) in PPCS participants who completed the six-week aerobic exercise and stretching protocols at baseline and follow-up. Eleven participants [ACC: aerobic (n = 7), stretching (n = 4); SM: aerobic (n = 8), stretching (n = 5)] completed the 6-week protocol and had data of sufficient quality to include in analysis.