2213
Utility of Residual Water in Proton MR Spectroscopy. The Measurement of Voxel Temperature and Hypoxia1Radiology, Stanford, Stanford, CA, United States, 2Electrical Engineering, Stanford, Stanford, CA, United States, 3Cardiothoracic Surgery, Stanford, Stanford, CA, United States
Synopsis
The basic mantra for in vivo MR spectroscopy is to look beyond the dominant water and lipid signals. Although water is very well characterized by MRI, partially suppressed water can provide unique information in proton MR spectra. In the results presented, residual water-by-design strategy is demonstrated to extend beyond use as a reference. For the protocol described, water derived measurements of temperature and hypoxia, add critical value at no compromise.
Introduction
Water, the dominant proton MR spectroscopy signal observed in vivo, is normally suppressed to avoid interference with the much smaller metabolite signals. Complete suppression of water, to the level of the metabolites or lower, is a recent consensus recommendation for in vivo brain spectra (1,2). However, treating water as a nuisance that needs to be completely removed in acquisition ignores the potential value of this signal. Collection of a separate unsuppressed water reference is only a partial solution. In this paper we characterize and explore the utility of a dominant but manageable residual water signal. Manageable residual water is defined here as a signal that can be removed post acquisition without compromise to the baseline or to the spectral quality of the remaining metabolites. The use of strong residual water for phase/frequency correction (3) and for optimal coil combination (4) are well known, but may be replaced, at good SNR, by the N-acetyl signal of NAA. Two properties of residual water that are difficult to replace, however, are its chemical shift and line-shape. Water chemical shift, referenced to the N-acetyl of NAA, can be used a measure of voxel temperature (5). Residual water line-shape is sensitive to the level of vascular deoxy-hemoglobin (6), and therefore brain hypoxia. Changes to the water integral are also preserved in the residual and may be useful. Unnecessary removal of this information during data acquisition is hard to justify. A better recommendation relative to water is to leave 1-5% of the water signal by design. This approach also avoids the undesirable addition of scan time for optimization of water suppression, and the possible baseline impact of over-rotation of a small metabolite-level residual water signal. At 1-5% residual water, impact on the spectral quality of the remaining peaks can be avoided, especially once water is removed with simple post processing methods.This work is part of range finding directed at establishing a 3T MR protocol to study the acute neurological impact of cardiopulmonary bypass using a piglet model (7). Temperature, blood chemistry and the fundamental bypass method itself can impact the metabolic stress that we hope to quantify before, during and after on-pump circulatory arrest. Although these examples are from a specific metabolic application for MR spectroscopy, we believe the general principles of water suppression strategy are more general. Retaining a modest residual water signal should be considered during any MR spectroscopy protocol design.Methods
Range-finding studies of 18 piglets weighing 5 – 14 kg on cardiopulmonary bypass pump, were conducted as previously described (7). Piglets were placed in a GE MR750 scanner (GE Healthcare, Waukesha, WI) equipped with an 8-channel array “knee” coil. Localizer T1 FSE images were acquired, and a 12 x 12 x 15 mm3 right midbrain voxel was prescribed for either a GE Healthcare WIP modified PRESS, or sLASER sequence. Data were collected at TE 30 or TE 35 at TR 2000. The TE Average (2DJ) option was also used for some of the samples at a TEA 168 and TR 1616, 2000 and 6000 to explore added resolution and to capture T1 and T2 changes. Spectra of 128 or 256 averages were acquired with 5000 Hz bandwidth and either 2K or 4K points. The physiological range included temperatures between 15°C and 37°C, with baseline spectra as well as spectra during circulatory arrest and recovery. Blood glucose was also manipulated. Preprocessing included pure water subtraction to achieve a baseline neutral residual water less than 5x metabolite level.Results
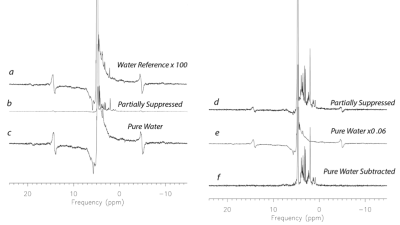
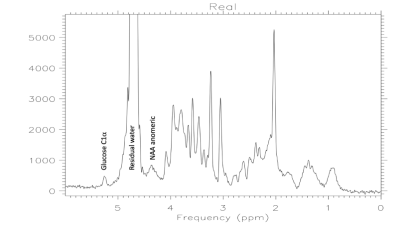
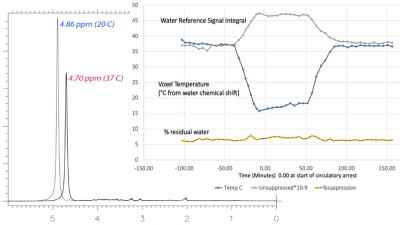
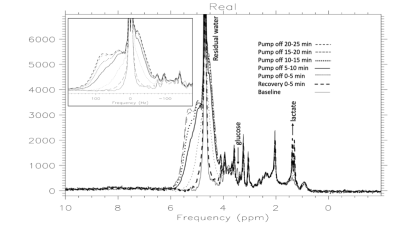
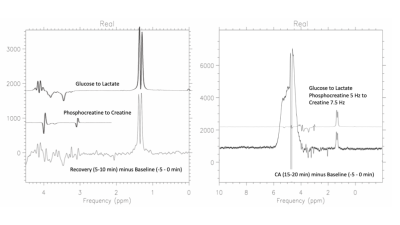
Figure 1 illustrates the pure water subtraction process used for spectral data containing a large residual water signal. The artifacts scale with the water resonance as observed in figure 1a and 1d. Artifacts drop below the noise floor after subtraction. As illustrated in figure 2, at 1% residual water, peaks near water such as the C1α of glucose and anomeric proton of NAA retain good fidelity. Baseline does not appear to be impacted. As shown in Figure 3, water signal is shifted downfield and increases linearly with decreasing temperature. The signal increase is reflected in the partially suppressed water signal, yielding a constant % residual. Voxel temperature measurement avoids an overshoot of up to 5°C that we observed when using only rectal temperatures for pump feedback. Figure 4 is an example of the low-level water line-shape changes observed during circulatory arrest. The “powder-pattern” shape is consistent with deoxy-hemoglobin levels as previously described (6). Assuming linear correlation between powder-pattern width and oxygen levels, it should be possible to extract voxel oxygen saturation levels. Figure 5 shows the use of spectral difference to capture the low-level line-shape effects caused by circulatory arrest without the need for a water reference and pure water subtraction.Discussion
The examples from this range finding study have convinced us of the value of a residual water signal as part of our proposed study design. When it comes to water signal in MR spectroscopy, there is no reason to be hydrophobic.Acknowledgements
NIH P41EB015891, R01MH110683References
1. Near, J., Harris, A. D., Juchem, C., Kreis, R., Marjanska, M., Oz, G., Slotboom, J., Wilson, M., and Gasparovic, C. (2020) Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts' consensus recommendations. NMR Biomed, e42572.
2. Oz, G., Deelchand, D. K., Wijnen, J. P., Mlynarik, V., Xin, L., Mekle, R., Noeske, R., Scheenen, T. W. J., Tkac, I., and Experts' Working Group on Advanced Single Voxel, H. M. (2020) Advanced single voxel (1) H magnetic resonance spectroscopy techniques in humans: Experts' consensus recommendations. NMR Biomed, e42363.
3. Webb, P. G., Sailasuta, N., Kohler, S. J., Raidy, T., Moats, R. A., and Hurd, R. E. (1994) Automated single-voxel proton MRS: technical development and multisite verification. Magn Reson Med 31, 365-3734.
4. An, L., Willem van der Veen, J., Li, S., Thomasson, D. M., and Shen, J. (2013) Combination of multichannel single-voxel MRS signals using generalized least squares. J Magn Reson Imaging 37, 1445-14505.
5. Corbett, R. J., Laptook, A. R., Tollefsbol, G., and Kim, B. (1995) Validation of a noninvasive method to measure brain temperature in vivo using 1H NMR spectroscopy. J Neurochem 64, 1224-12306.
6. Springer, C. S., Patlack, C.S., Palyka, I, and Huang W. (1999) Principles of Susceptibility Contrast-Based Functional MRI: The Sign of the Functional MRI Response. in Functional MRI (Moonen, C. T. W., and Bandettini, P.A ed.), Springer-Verlag, Berlin. pp 91-1027.
7. Hanley, F. L., Ito, H., Gu, M., Hurd, R., Riemer, R. K., and Spielman, D. (2020) Comparison of dynamic brain metabolism during antegrade cerebral perfusion versus deep hypothermic circulatory arrest using proton magnetic resonance spectroscopy. J Thorac Cardiovasc Surg 160, e225-e227
Figures