2204
Reduced fMRI activation in the fusiform face area is related to higher hallucination proneness and lower glutamate levels assessed by 1H MRS1Center for Stroke Research Berlin, Charité – Universitätsmedizin Berlin, Berlin, Germany, 2Department of Psychiatry and Psychotherapy, Charité – Universitätsmedizin Berlin, Berlin, Germany
Synopsis
The neurophysiological and neurochemical alterations involved in the formation of hallucinations are not sufficiently understood. fMRI was used during a face detection task, and neurotransmitter levels in the visual cortex were measured by 1H MRS at 3T to elucidate processes involved in the false (hallucinatory) detection of faces in pure noise patterns. Increased hallucinatory face detections were related to decreased activation of the fusiform face area. In addition, decreased face-dependent activation was related to reduced glutamate levels. These findings substantiate theories of hallucinatory misperceptions, which implicate impaired glutamatergic transmission in a reduced ability to differentiate between meaningful information and noise.
Introduction
Schizophrenia is a serious mental illness with poorly understood etiology1. Disease manifestations comprise among others psychotic symptoms, such as delusions and hallucinations. The "Psychosis Continuum" theory postulates that clinical manifestations of psychosis represent the most extreme form of psychosis proneness, which is relatively continuously distributed in the general population2. Importantly, it implies similar etiological mechanisms involved in the formation of subclinical and clinical psychotic symptoms. Hence, the investigation of mechanisms involved in psychotic symptoms in non-patient populations might contribute to explaining clinical symptoms as well. A subclinical hallucination proneness has been assessed with tasks measuring the tendency to detect meaning in noise stimuli. Here, schizophrenia patients, particularly those having hallucinations, show an increased tendency to recognize meaningful information in noise3, 4. Moreover, increased detection in noise has been related to trait hallucination proneness assessed by questionnaires in non-clinical populations5,6. Despite intense research efforts, the neurochemical and neurofunctional underpinnings of hallucinations are not sufficiently understood. Increased dopaminergic and reduced glutamatergic neurotransmission have been hypothesized based on an abundancy of experimental designs including postmortem pathological examination, pharmacological challenging, PET, and magnetic resonance spectroscopy (MRS)7. In this study, functional MRI (fMRI) and MRS were used to identify correlates of hallucination proneness in healthy individuals.Methods
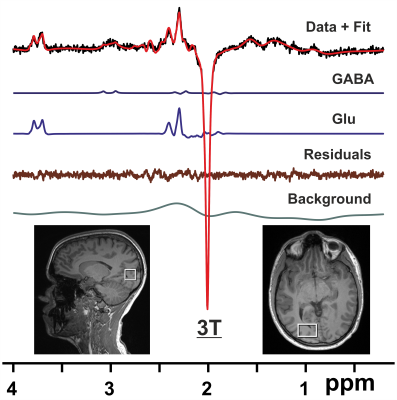
Subjects and Hardware: Eighteen subjects (aged 25 – 48 yrs, 11 f) were included (two subjects did not have MRS measurements for technical reasons). Scans were performed on a 3T Trio system (Siemens Healthineers, Erlangen, Germany) using a 32 channel radiofrequency (RF) coil (N = 9), and after an upgrade on a 3T PrismaFit system employing a 64 channel RF coil (N = 9).MRS: 1H MRS was performed as described previously8 including localized RF calibration and vendor-supplied shimming. Single volume spectra from the right visual cortex were acquired using the MEGA-PRESS technique9 (VOI = 20x30x20 mm, TR/TE = 3000/68 ms, number of averages = 128, Tacq = 1024 ms, and editing pulse at 1.9 ppm). The FID-A toolkit10 was used for pre-processing of MRS data. Resulting spectra were analyzed using LCModel11 with a simulated basis set.
Face task: To quantify psychosis-like mispercepts of illusory faces in noise, a face detection task that required the participants to detect faces embedded in noise was devised6. The face task comprised 9 runs each with 40 stimuli, of which 20 contained a face and 20 contained no face (noise patterns only). Stimuli were presented for 4000 ms. After that, participants were instructed to indicate if they had recognized a face (2000 ms) followed by an inter-trial-interval (fixation cross) jittered around 2500 ms. The percentage of false alarms (detection of faces in pure noise) was computed as a measure of hallucination proneness.
FMRI acquisition and analysis: During the face task, functional images were recorded using single-shot gradient-echo EPI (TR = 2250 ms, TE = 25 ms, voxel size = 2.5 mm isotropic). Preprocessing and analysis were performed using the statistical parametric mapping (SPM12) software. Preprocessing comprised slice timing, SPM12 standard realignment and unwarping including field map correction, co-registration, normalization to MNI space, and spatial smoothing. On single subject level, brain activation differences related to presence of faces in the stimuli were analyzed using a general linear model. The blood oxygen level dependent response was modeled by a canonical hemodynamic response function for face cues, no face cues and button-presses and ratings as noise regressors. The resulting t-statistic images with face-dependent effects (face cues versus no face cues) were submitted to group level analysis. At group level, main effects of face versus no face were were tested. In addition, correlations between neurotransmitter levels assessed by MRS and face-dependent effects in fMRI as well as correlations between false alarms in the face task and face-dependent effects were evaluated. For small volume correction of significance levels with family wise error (FWE), a ROI of the bilateral gyrus fusiformis (face area) was used.
Results
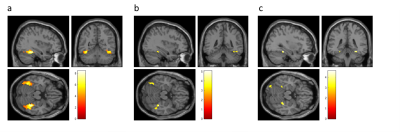
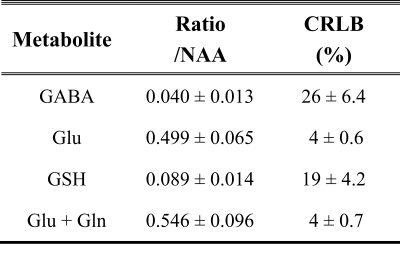
Shimming resulted in water linewidths of 7.1 ± 0.6 Hz across all MRS scans. Analysis of MEGA-PRESS difference spectra (Fig. 1) allowed quantification of neurotransmitters GABA, glutamate (Glu), glutathione (GSH), and combined Glu + glutamine (Gln) with respect to N-acetylaspartate (NAA) (Table 1). Evaluating fMRI data revealed that stimuli with faces compared to those without faces led to significantly increased activation in the gyrus fusiformis known to be a face-processing area (Fig. 2a). The magnitude of this face-dependent activation was decreased in individuals with more false alarms (hallucination proneness) in the face task (Fig. 2b). Importantly, decreased Glu/NAA ratios in the right visual cortex were also associated with decreased face-dependent activation (Fig. 2c). No correlation of fMRI results with GABA was found.Discussion
In this study, neurochemical and neurofunctional correlates of visual hallucination proneness (defined as an increased readiness to detect faces in pure noise patterns) were investigated. Decreased face-dependent fMRI activation in the fusiform face area (FFA) was associated with increased hallucination proneness. Moreover, using MRS it was shown that decreased face-dependent activation in the FFA was related to lower glutamate levels. These findings further substantiate theories that imply impaired glutamatergic neurotransmission and a decreased ability to distinguish between noise and meaningful perceptual information in the pathogenesis of hallucinations.Acknowledgements
No acknowledgement found.References
1. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers 2015;1:15067.
2. DeRosse P, Karlsgodt KH. Examining the Psychosis Continuum. Curr Behav Neurosci Rep 2015;2:80-89.
3. Bentall RP, Slade PD. Reality testing and auditory hallucinations: a signal detection analysis. The British journal of clinical psychology 1985;24 ( Pt 3):159-169.
4. Powers AR, Mathys C, Corlett PR. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 2017;357:596-600.
5. Partos TR, Cropper SJ, Rawlings D. You Don't See What I See: Individual Differences in the Perception of Meaning from Visual Stimuli. PloS one 2016;11:e0150615.
6. Stuke H, Kress E, Weilnhammer VA, Sterzer P, Schmack K. Overly strong priors for socially meaningful visual signals in psychosis proneness. bioRxiv 2018:473421.
7. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 2015;29:97-115.
8. Mekle R, Schmack K, Fiebach JB, Stuke H. Pharmacologically increased dopamine levels reduce GABA and glutamate concentrations in visual brain areas – a 1H MRS study at 3T. ISMRM 27th Annual Meeting. Montreal, QC, Canada: International Society of Magnetic Resonance in Medicine, 2019: 2257.
9. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine 1998;11:266-272.
10. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med 2017;77:23-33.
11. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672-679.
Figures