2202
Associations of tau aggregates and oxidative stress to apathy levels in progressive supranuclear palsy
Kiwamu Matsuoka1,2, Yuhei Takado1, Kenji Tagai1, Manabu Kubota3, Yasunori Sano1, Keisuke Takahata1, Maiko Ono1, Chie Seki1, Hideki Matsumoto1,4, Hironobu Endo1, Hitoshi Shinotoh1, Jamie Near5, Kazunori Kawamura1, Ming-Rong Zhang1, Hitoshi Shimada1, and Makoto Higuchi1
1National Institute of Radiological Sciences, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 2Department of Psychiatry, Nara Medical University, Kashihara, Japan, 3Department of Psychiatry, Kyoto University Graduate School of Medicine, Kyoto, Japan, 4Department of Oral and Maxillofacial Radiology, Tokyo Dental College, the city of Chiyoda-ku, Japan, 5Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montréal, QC, Canada
1National Institute of Radiological Sciences, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan, 2Department of Psychiatry, Nara Medical University, Kashihara, Japan, 3Department of Psychiatry, Kyoto University Graduate School of Medicine, Kyoto, Japan, 4Department of Oral and Maxillofacial Radiology, Tokyo Dental College, the city of Chiyoda-ku, Japan, 5Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montréal, QC, Canada
Synopsis
Apathy is characterized by lack of motivation. We investigated the mechanisms underlying apathy in progressive supranuclear palsy (PSP), which is characterized by tau aggregate accumulations causing oxidative stress in the brain. Using magnetic resonance spectroscopy and tau positron emission tomography, we found associations of apathy levels with glutathione levels in the posterior cingulate cortex (PCC), tau aggregate accumulation levels in the angular gyrus/PCC, and atrophy of the right inferior frontal gyrus and anterior cingulate cortex. The vulnerability of the anterior and posterior brain regions where apathy is related is suggested as possible underlying mechanisms for apathy in PSP.
INTRODUCTION
Progressive supranuclear palsy (PSP) is one of the primary tauopathies. 1 More than half of patients with PSP present apathy, defined by lack of motivation. 2 While we previously reported that apathy levels are associated with tau aggregates in the anterior brain regions in Alzheimer’s disease 3, the underlying mechanism of apathy in relation to tau aggregates in PSP patients remains unknown. We hypothesized that tau aggregates cause oxidative stress (OS), leading to the vulnerability of brain regions where apathy is related. To test this hypothesis, we performed positron emission tomography (PET) scans with a tau ligand, 18F-PM-PBB3 4, magnetic resonance spectroscopy (MRS) scans to measure levels of the antioxidant glutathione (GSH) 5, and volumetry.METHODS
We enrolled 20 PSP patients and 23 healthy controls (HCs) confirmed to be amyloid-negative. We performed MRS using a short TE spin-echo full-intensity acquired localized single voxel spectroscopy (SPECIAL) sequence (TR/TE/number of excitations = 3000 ms / 8.5 ms / 128), 18F-PM-PBB3 PET scans, and examined three-dimensional T1-weighted images. Given the importance of both the anterior and posterior cingulate cortex for apathy and GSH 6, 7, volumes of interest for MRS were manually placed on the anterior cingulate cortex (ACC, 30 × 20 × 20 mm2) and posterior cingulate cortex (PCC, 20 × 20 × 20 mm2) regions (Figure 1). We analyzed MRS data using LCModel software for a linear combination of model fitting with a basis set including GSH and corrected metabolite values for CSF partial volumes. We generated standardized uptake value ratio (SUVR) images of 18F-PM-PBB3 PET with cerebellar gray matter (GM) as a reference, using data at 90-110 min. We performed voxel-based morphometry (VBM) about the associations of apathy scale (AS) scores with 18F-PM-PBB3 SUVRs and GM volumes in PSP patients. T-tests, χ2-tests, and analysis of covariance were used to compare the two groups and Pearson partial correlation and Spearman's partial rank-order correlation were used to evaluate the associations of AS scores. Path analysis was used for models explaining apathy. This study was approved by the Certified Review Board.RESULTS
The demographic profiles were summarized. MRS: The average Cramér–Rao lower bound of GSH measurements were 6.1% and 6.9% in ACC and PCC, respectively. While there was no significant difference between the PCC GSH levels of PSP patients and HCs (p = 0.87), partial correlation analyses with age and PSP Rating Scale (PSPRS) scores showed negative correlations between the PCC GSH levels and AS scores (r = -0.62, p = 0.014) (Figure 2a). The GSH levels in the ACC in PSP patients did not show significant associations with AS scores (p = 0.87). 18F-PM-PBB3 PET: In PSP patients, a positive association was found between AS scores and 18F-PM-PBB3 SUVRs in the bilateral angular gyrus (AG) and PCC using age and PSPRS scores as nuisance variables (Figure 2b and 3). We found significantly higher 18F-PM-PBB3 SUVRs in the bilateral AG in PSP patients compared with HCs using age as nuisance variables (the left AG: p = 0.006; the right AG: p = 0.004). Partial correlation analyses with age and PSPRS scores suggested that there were negative correlations of the GSH levels in the PCC with 18F-PM-PBB3 SUVRs in the PCC and AG (the PCC: r = -0.63, p = 0.012; the AG: r = -0.64, p = 0.010) (Figure 2c-d). Volumetry: In the VBM using age, PSPRS scores, and TBV as nuisance variables in PSP patients, we found negative associations between AS scores and the GM volumes in the right inferior frontal gyrus (IFG) and ACC (Figure 4). Path analysis: Path analysis generated a model between the AS scores, the 18F-PM-PBB3 SUVRs in the AG, the GSH levels in the PCC, and the GM volumes in the right IFG satisfied the criteria for a good fit (χ2 (2) = 2.07, p = 0.36, CFI = 1.00, NFI = 0.91, RMSEA = 0.046) (Figure 5).DISCUSSION and CONCLUSION
We found associations of apathy with tau accumulation levels in the AG/PCC and GSH levels in the PCC, in addition to atrophy of the right IFG and ACC, which have key roles in the reward system closely related to apathy 8. Because the AG and PCC have been reported to be associated with action initiation 9 and self-reference 10, respectively, the deterioration of these functions might also be related to apathy. 7 We also found negative associations between 18F-PM-PBB3 SUVRs and GSH levels, suggesting a link between tau aggregate accumulations and OS, in line with previous evidence that aggregates initiate inflammation which causes reactive oxygen species production. 11, 12 Our model generated by path analysis suggests that the depletion of GSH in the PCC is associated with abnormal tau aggregation in the AG and PCC region. Antioxidant GSH might serve as the resilience factor of the neural system by limiting the damage from OS caused by tau aggregate accumulation. 13 In conclusion, these findings suggest a potential link between tau aggregates, OS, and brain atrophy in both the anterior and posterior brain regions as underlying brain mechanisms for apathy in PSP patients.Acknowledgements
The authors thank all patients and their caregivers for participation in this study, clinical research coordinators, PET and MRI operators, and research ethics advisers at QST for their assistance to the current projects.References
- Steele JC, Richardson JC, and Olszewski J, Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum With Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch Neurol., 1964. 10: 333-59.
- Borroni B, Alberici A, Agosti C, et al., Pattern of behavioral disturbances in corticobasal degeneration syndrome and progressive supranuclear palsy. Int Psychogeriatr, 2009. 21: 463-8.
- Kitamura S, Shimada H, Niwa F, et al., Tau-induced focal neurotoxicity and network disruption related to apathy in Alzheimer's disease. J Neurol Neurosurg Psychiatry, 2018. 89: 1208-1214.
- Tagai K, Ono M, Kubota M, et al., High-Contrast In Vivo Imaging of Tau Pathologies in Alzheimer's and Non-Alzheimer's Disease Tauopathies. Neuron, 2020.
- Xin L, Mekle R, Fournier M, et al., Genetic Polymorphism Associated Prefrontal Glutathione and Its Coupling With Brain Glutamate and Peripheral Redox Status in Early Psychosis. Schizophr Bull, 2016. 42: 1185-96.
- Shukla D, Mandal PK, Tripathi M, et al., Quantitation of in vivo brain glutathione conformers in cingulate cortex among age-matched control, MCI, and AD patients using MEGA-PRESS. Hum Brain Mapp, 2020. 41: 194-217.
- Tumati S, Martens S, de Jong BM, et al., Lateral parietal cortex in the generation of behavior: Implications for apathy. Prog Neurobiol, 2019. 175: 20-34.
- Le Heron C, Holroyd CB, Salamone J, et al., Brain mechanisms underlying apathy. J Neurol Neurosurg Psychiatry, 2019. 90: 302-312.
- Geschwind N, Disconnexion syndromes in animals and man. I. Brain, 1965. 88: 237-94.
- Northoff G, Heinzel A, de Greck M, et al., Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage, 2006. 31: 440-57.
- Perea JR, Bolós M, and Avila J, Microglia in Alzheimer's Disease in the Context of Tau Pathology. Biomolecules, 2020. 10: 1439.
- Simpson DSA and Oliver PL, ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel), 2020. 9: 743.
- de Roos B and Duthie GG, Role of dietary pro-oxidants in the maintenance of health and resilience to oxidative stress. Mol Nutr Food Res, 2015. 59: 1229-48.
Figures
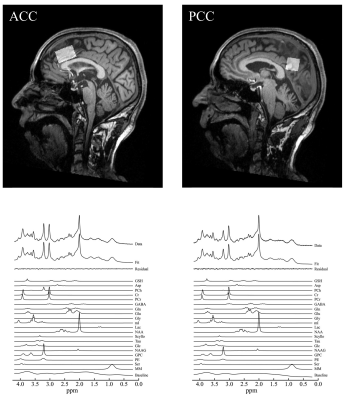
Representative
spectrum in the ACC and PCC. Volumes of interest of magnetic
resonance spectrum were placed at the ACC and PCC. A representative
MRS acquired with the SPECIAL sequence, the corresponding LCModel spectral fit,
fit residual, macromolecules (MM), baseline and individual metabolite fits
including GSH in the ACC and PCC.
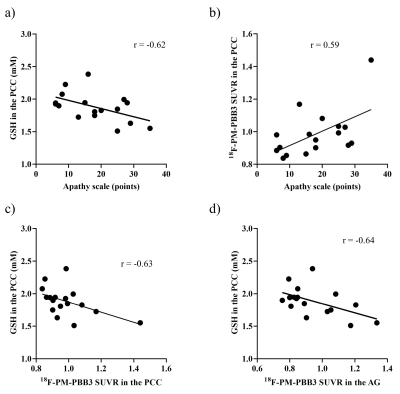
Scatterplots
presented
the association of apathy scale scores with the GSH levels (a) and 18F-PM-PBB3
SUVRs (b) in the PCC, and the association of the GSH levels in the PCC and 18F-PM-PBB3
SUVRs in the PCC (c) and the AG (d) in patients with PSP.
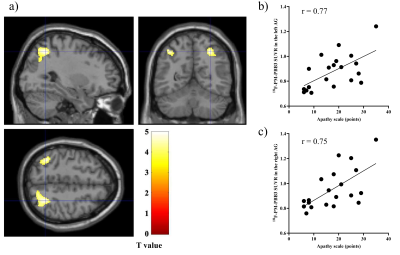
a) Sagittal,
coronal, and transverse brain views showed voxels with positive associations between apathy scale scores and 18F-PM-PBB3
SUVRs
in patients with PSP after controlling for the effects of age and PSPRS. b, c)
Scatterplots showing the association between apathy scale scores and 18F-PM-PBB3
SUVRs in the detected areas (AG).
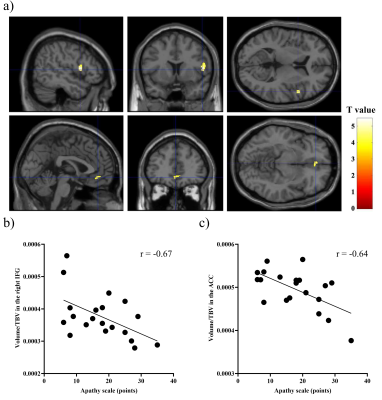
a) Brain views showed voxels with positive associations between apathy scale
scores and GM volumes in patients with PSP after
controlling for the effects of age, PSPRS, and total brain volume (TBV) (upper: the right inferior frontal
gyrus, lower: anterior cingulate cortex). b, c) Scatterplots showed the association between apathy scale scores and regional GM volumes divided by the
corresponding subject’s TBV.
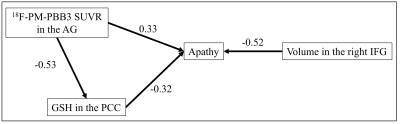
A path analysis model
of the relationship between the apathy scale scores, the 18F-PM-PBB3 SUVRs
in the AG, the GSH concentration in the PCC, and the GM
volumes in the right IFG divided by the corresponding subject’s TBV. The standardized regression weights are
shown for the path analysis model.