2194
Generalizing Ultra-low-dose PET/MRI Networks Across Radiotracers: From Amyloid to Tau1Radiology, Stanford University, Stanford, CA, United States, 2Salem State University, Salem, MA, United States, 3Neurology and Neurological Sciences, Stanford University, Stanford, CA, United States
Synopsis
We have previously trained a deep learning network with simultaneous PET/MRI inputs to generate diagnostic quality images from ultra-low-dose amyloid PET acquisitions. With data bias being a known issue in deep learning-based applications, we aim to investigate whether this network could generalize to ultra-low-dose tau PET image enhancement. Results of this study show that data bias across radiotracers needs to be accounted for before applying an ultra-low-dose network trained on one tracer to another.
Introduction
Simultaneous PET/MRI provides complementary morphological and functional information; moreover, with perfect spatial and temporal registration of the two imaging datasets, the information derived from one modality can assist image processing of the other1. In the case of dementia patients, simultaneous PET/MRI provides the opportunity of a “one-stop shop” imaging exam to obtain information for the most important biomarkers: amyloid deposition, pathologic tau, and neurodegeneration2. However, radiation exposure related to the radiotracers administered to imaging subjects will limit widespread screening in cognitively normal subjects as well as the amount of follow-up evaluations due to radiation dose thresholds.With the advent of convolutional neural networks (CNNs) and increased computing power, it is now possible to use deep learning methods that incorporate spatially correlated multimodal MRI and PET information to produce high quality PET images from scan protocols with markedly reduced injected radiotracer dose. Previously, we have demonstrated the ability to reduce radiotracer dose by 100-fold using these approaches for 18F-florbetaben amyloid studies3. However, with data bias from the training set, whether such methods are transferable to data collected on a different scanner4 or a different tracer altogether warrants further investigation. Here, we investigate whether the network trained previously generalizes to acquisitions using the second-generation tau radiotracer 18F-PI-26205. This technique can potentially increase the utility of hybrid PET/MR imaging in clinical diagnoses and longitudinal studies.
Methods
PET/MR data acquisition32 participants (19 female, 68.2±7.1 years) were recruited for the ultra-low-dose amyloid network; 328±32 MBq of the amyloid radiotracer 18F-florbetaben was injected. 44 participants (18 female, 70.2±7.5 years) were recruited for the ultra-low-dose tau network; 221±61 MBq of the tau radiotracer 18F-PI-2620 was injected. MRI (T1-, T2-weighted, and T2-FLAIR for amyloid participants; T1-weighted, GRE, and T2-FLAIR for tau participants) and PET data (90-110 and 60-90 minutes post-injection for amyloid and tau, respectively) were simultaneously acquired on an integrated PET/MRI scanner (SIGNA PET/MR, GE Healthcare). The raw list-mode PET data were reconstructed for the full-dose ground truth image and were also randomly undersampled by a factor of 100 (amyloid, 1%) or 20 (tau, 5%) and then reconstructed to produce an ultra-low-dose PET image.
CNN implementation
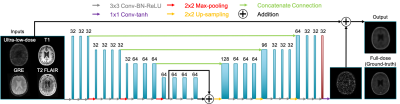
The ultra-low-dose amyloid network (“Amyloid Network”) was trained with the proposed structure in Chen et al. (Figure 1)3. Using the same structure for the tau data, network training was either done from scratch on tau images (“Tau Network”) or used the weights derived from the Amyloid Network, with the last layer fine-tuned on the tau images (“Fine-tuned Network”). The tau data were also directly used as inputs to the Amyloid Network. 4-fold cross-validation was used to efficiently utilize all datasets and to match the number of training sets for the amyloid network (33 for training, 11 for testing per fold).
Data analysis
A FreeSurfer-derived brain mask for each participant was used for voxel-based analyses. For each axial slice of the volumes, the image quality of the enhanced PET and the ultra-low-dose PET images within the brain mask was compared to the original full-dose image using the metrics peak signal-to-noise ratio (PSNR), structural similarity (SSIM), and root-mean-squared error (RMSE). The values were averaged across slices to derive a set of metrics per participant. Paired t-tests at the p=0.05 level were used to compare the metrics derived from different image types.
Results and Discussion
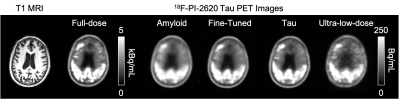
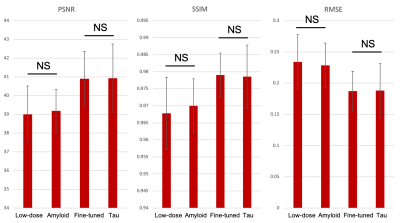
Qualitatively, the synthesized tau images showed marked improvement in noise reduction compared to the ultra-low-dose image. The results directly obtained from the Amyloid Network were blurrier than the other image types, while the two other outputs (Fine-tuned and Tau Networks) better resemble the ground truth image and were more able to reflect the underlying anatomy in tau uptake (Figure 3).Quantitatively, the images synthesized from the Fine-tuned Network and the Tau Network have similar metrics (p>0.60 for all comparisons) while significantly outperforming the ultra-low-dose images (p<0.001 for all comparisons). The ultra-low-dose images performed the worst in all metrics on average, but similar to the outputs directly obtained from the Amyloid Network (p>0.05 for all comparisons). This shows that data bias related to the uptake patterns in different PET radiotracers should be accounted for before performing the enhancement of ultra-low-dose images.
Conclusion
This work has shown that high-quality tau PET images can be generated using deep learning methods with simultaneously-acquired MR images and ultra-low-dose PET data, but that it is important to either re-train or fine-tune networks using tracer-specific data.Acknowledgements
This project was made possible by NIH P41-EB015891, R01-EB025220-03W1, the Stanford Alzheimer’s Disease Research Center, GE Healthcare, and Life Molecular Imaging.References
1. Catana C, Drzezga A, Heiss WD, Rosen BR. PET/MRI for neurologic applications. J Nucl Med. 2012;53(12):1916-1925.
2. Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562.
3. Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-Low-Dose (18)F-Florbetaben Amyloid PET Imaging Using Deep Learning with Multi-Contrast MRI Inputs. Radiology. 2019;290(3):649-656.
4. Chen KT, Schürer M, Ouyang J, et al. Generalization of Deep Learning Models for Ultra-low-count Amyloid PET/MRI using Transfer Learning. Eur J Nucl Med Mol Imaging. 2020.
5. Mueller A, Bullich S, Barret O, et al. Tau PET imaging with (18)F-PI-2620 in Patients with Alzheimer Disease and Healthy Controls: A First-in-Humans Study. J Nucl Med. 2020;61(6):911-919.
Figures