2192
Deep learning for fast 3D low field MRI1Center for Adaptable MRI Technology (AMT Center), Department of Biomedical Engineering, University of Basel, Allschwil, Switzerland
Synopsis
Low magnetic field (LF) MRI is currently gaining momentum as a complementary, more flexible and cost-effective approach to MRI diagnosis. However, the impaired Signal-to-Noise Ratio, leading in turn to prolonged acquisition times, challenges its relevance at the clinical level. Recently, reconstructing an alias-free image using deep learning techniques has shown promising results. In this study, we leverage deep learning reconstruction to demonstrate the feasibility of highly undersampled (20% sampling) 3D LF MRI at 0.1 T. The model performance has been evaluated on both retrospective and acquired, prospective 3D LF data.
Introduction
Low magnetic field (LF) MRI is currently gaining momentum as a complementary, adaptable approach to MRI diagnosis1. However, the further impaired Signal-to-Noise Ratio (SNR), leading in turn to prolonged acquisition times, challenges its relevance at the clinical level. MRI sequences can be accelerated from undersampling strategies, but large undersampling factors can cause artefacts, such as blurring and severe aliasing when relying on the typical Fourier formalism. Recently, reconstructing an alias-free image using deep learning techniques has shown promising results2,3. In this study, we leverage deep learning reconstruction to demonstrate the feasibility of highly undersampled (20% sampling) 3D LF MRI at 0.1 T (4.25 MHz). The model performance is evaluated on both retrospective and acquired, prospective 3D LF data.Material and Method
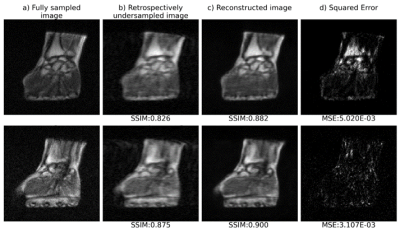
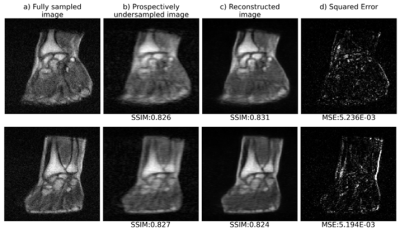
A total of 19 sets of fully sampled, 3D spoiled gradient echo (GRE) MR images of the human wrist and hand were acquired at 0.1 T using a single Tx/Rx coil. Ten of the latter sets were acquired using the following parameters: matrix = 128 × 115 × 9, voxel size = 1.2 × 1.2 × 6.3 mm3, TE/TR = 7.2/31 ms, number of averages = 28 (acquisition time = 14 min 56 s). The other 9 sets were collected using heterogenous sets of sequence parameters in order to expand the size of our dataset, and overcome overfitting issues. In addition, data augmentation was applied using a 3D version of the data generator function from the Keras library4, capable of handling 3D image processing, including image flipping, shifting and rotation. k-space undersampling was performed on both the second and third phase encode directions (ky and kz) using a Gaussian mask where 20 % of the data was retained. Full and undersampled 3D k-spaces were resized in x and y-axes to reach a matrix size of 128 x 128. The full and undersampled 3D k-spaces were then inverse Fourier transformed, and normalized. It is important to mention that the reconstruction pipeline is a 2D approach (x-y axes), each “slice” being considered as one individual input image.U-net5 was adopted as a convolutional neural network architecture and trained using the RMSProp optimizer with an adaptive learning rate. Network performance was assessed on 2 retrospective testing sets. Mean Squared Error (MSE) and Structure Similarity Index (SSIM) were chosen as evaluation metrics. Our model performance was finally evaluated on prospective undersampled images with the protocol and sampling scheme described above; the total acquisition time was 3 min 7 s.
Results
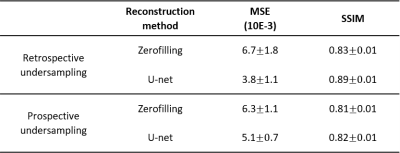
Fig.1 and 2 show respectively the reconstruction of retrospectively and prospectively undersampled data. As can be seen, the structures, the contrast, and the sharpness are mostly preserved in the reconstructed images. Quantitative results are summarized in table 1. The mean SSIM and MSE values support the effectiveness of U-net reconstruction in both retrospective and prospective experiments.Discussion and conclusion
Despite the small and heterogenous LF MR training dataset, quantitative results on both retrospectively and prospectively accelerated acquisitions show that our approach is able to reconstruct 5-fold undersampled 3D LF MR data while maintaining anatomical structure, and preserving contrast. This could be a significant step towards clinical relevance of LF MRI. Future work will focus on improving the model performance by expanding the training dataset and further preserving high spatial frequencies, as well as using coils better suited for hand/wrist applications along with high performance imaging sequences.Acknowledgements
- The Swiss National Science Foundation Grant No. PP00P2_170575
- The Swiss National Science Foundation Grant No. PCEFP2_186861
- The University of Basel Faculty of Medicine
References
1Sarracanie et al. Front Phys 8:172 (2020)
2Hammernik et al. Magn Reson Med, 79(6): 3055–3071 (2018)
3Schlemper et al. IEEE T Med Imaging (2018)
4https://keras.io/
5Ronneberger et al. arXiv:1505.04597, MICCAI (2015)
Figures