2190
Unsupervised Denoising for Low-field Diffusion MRI1Hyperfine Research Inc., Guilford, CT, United States, 2New York University, New York, NY, United States, 3Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
An unsupervised deep learning framework is proposed for denoising low-field 64 mT diffusion-weighted MRI images (DWI). The denoised DWI (b-value = 890 s/mm2) and apparent diffusion coefficient (ADC) maps were evaluated in a user study by four expert graders in terms of sharpness, noise reduction, and overall utility. Our framework was found to enable low-field DWI restoration with strong noise while maintaining relevant image features. 62.50% and 64.28% of processed images were rated clearly/far better overall for DWI and ADC, respectively, with only 0.05% of processed DWI and 0% of processed ADC rated clearly/far worse.
Background
Diffusion-weighted magnetic resonance imaging (DWI) is a key modality for clinical neuroimaging, used for detecting and staging anomalies such as stroke or tumours. Recently, Hyperfine Bedside MRI has demonstrated the utility of beside low-field MR imaging1,2, including DWI. However, due to image acquisition physics, it is challenging to obtain sufficient resolution and signal-to-noise (SNR) at low-field (64mT). Improved image quality can improve diagnostic confidence and effectiveness on downstream tasks, motivating the need for restoration algorithms that can simultaneously reduce noise levels while preserving important image features.While traditional denoisers such as BM3D3 remain competitive, they often result in over-smoothing or require manual image-specific parameter tuning. Recently, unsupervised deep networks have achieved remarkable success in reference-free denoising.4,5,6,7 However, they may assume pixel-wise independent identically distributed noise or repeat acquisitions with no motion. This does not hold for realistic MR acquisition, where images contain spatially-varying noise due to multi-coil acquisition and display spatial noise correlation with a Rician bias, all of which is more pronounced for low-field DWI. In this work, we propose a joint noise simulation and denoising approach inspired by Noiser2Noise7, which enables denoising correlated noise without ground truth, leading to improved DWI quality as measured by expert evaluation.
Materials and Methods
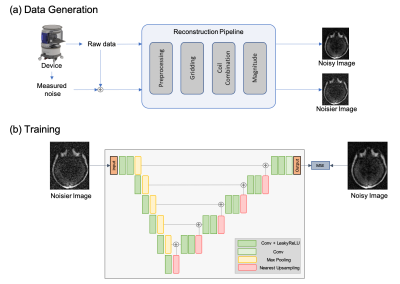
Imaging: A portable 64mT MRI scanner (Hyperfine SwoopTM) was deployed to the Neuro-ICU at Yale New Haven Hospital. Seven patients with neurological alterations were recruited and provided consent under a protocol approved by an institutional review board. The patients underwent a DWI imaging protocol (TE/TR = 24ms/34ms, 2x2x6mm3 resolution, b=890 s/mm2, scan time = 8 min, followed by b=0 s/mm2, scan time=1.5 min) using a 1Tx/8Rx coil. 15 healthy controls were recruited at Hyperfine research lab for additional imaging.Training: Given original noisy DWI images, Noisier2Noise works by regressing the original noisy image from a noisier image, where the additional noise is added based on a model-based noise simulator. The noise simulator adds measured system noise to the original noisy raw data and applies the image reconstruction pipeline to generate noisier images. The overall training data generation process is shown in Fig 1. The noisier image contained 10% higher noise compared to the original noisy image. Mean squared error (MSE) between the network output and the noisy image was minimized using Adam optimizer with learning rate 10-4.
Model Architecture: 2D 64x64 axial patches are used for training. The network architecture consists of a bias-free8 U-net (encoder-decoder architecture with skip connections) with 5 levels of downsampling. During the inference, the entire 3D image is fed slice-by-slice to the network and the output DWI is generated using post-hoc correction7.
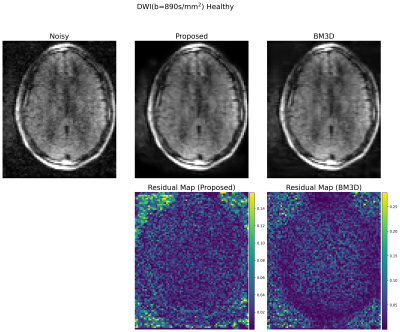
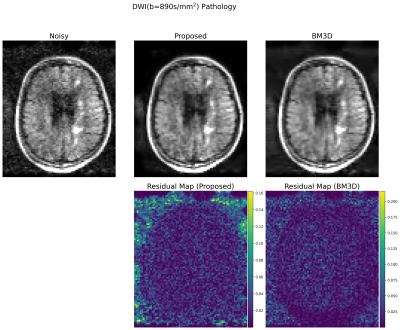
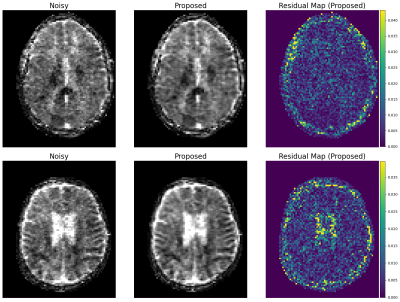
Evaluation: 22 DWI images with b=890s/mm2 and b=0s/mm2 were reconstructed using the trained denoiser. The performance was compared to the BM3D results, which were manually tuned for the optimal results. From the denoised images, clinically-relevant apparent diffusion coefficient (ADC) maps were created using the equation $$$ADC = - \ln ( x_b / x_0) / b$$$, where $$$x_b$$$ and $$$x_0$$$ are the denoised DWI images at b=890 and 0, respectively. Note that 8 cases with high levels of motion corruption were excluded from the subsequent study.
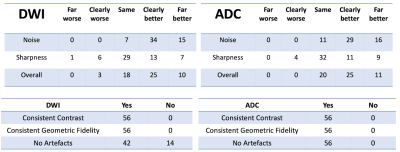
The unsupervised DWI denoising framework was then qualitatively evaluated by four expert graders with background in either MR physics, clinical science, and/or radiology. Images before and after denoising were shown to the raters as a side-by-side comparison. The users were asked to rate if the denoised image was “Far better”, “Clearly Better”, “Same”, “Clearly Worse”, or “Far Worse”, in terms of noise, sharpness and overall quality. The users were also asked binary questions if the denoised image had consistent image features as the input in terms of contrast, geometric fidelity, and whether artifacts were introduced.
Results
Example denoising results are shown in Fig 2-4. Results of the expert grading is summarized in Fig 5. In particular, at least 85% of the graders indicated “Same”, “Clearly Better”, “Far Better” for all categories. At least 80%, 35%, 60% voted “Clearly Better”, “Far better” for reduced noise, sharpness and overall quality, respectively. The denoised images also scored “Yes” for all consistency questions except for artifact, in which one grader noticed the blurring of the background artifact appearing structure-like. The reconstruction times were 0.06 ± 0.08 s for the proposed model on GPU and 5.58 ± 0.37 s for BM3D on CPU.Discussion and Conclusions
Our results demonstrate that unsupervised denoising via simulation and deep networks can reduce DWI noise while retaining image detail, sharpness, and overall quality. While BM3D also showed effectiveness, it tended to over-smooth the image and required subject and slice-specific parameter-tuning, making it challenging to deploy at clinical settings. The proposed deep learning approach demonstrated robustness to variable input noise, and was validated by scoring favorably from multiple expert graders for 14 images acquired at diverse conditions. The presented framework is effective as it does not require careful repetitive acquisition and can exploit large amounts of data available at clinical sites. As a drawback, the model can only remove noise similar in distribution to the simulations, limiting generalization to other image artifacts. Future work includes extending the model to compensate for additional artifacts that are difficult to simulate, such as motion and system imperfections.Acknowledgements
No acknowledgement found.References
Sheth, Kevin N., et al. "Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients." JAMA neurology. 2020.”
O’Halloran, Rafael, et al., “Bedside Stroke Imaging at 64mT”, ISMRM2020, Abstract #1799
Maggioni, Matteo, et al. "Nonlocal transform-domain filter for volumetric data denoising and reconstruction." IEEE Transactions on Image Processing. 2012.
Lehtinen, Jaakko, et al. "Noise2noise: Learning image restoration without clean data." International Conference on Machine Learning. 2018.
Krull, Alexander, Tim-Oliver Buchholz, and Florian Jug. "Noise2void-learning denoising from single noisy images." Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2019.
Batson, Joshua, and Loic Royer. "Noise2self: Blind denoising by self-supervision." International Conference on Machine Learning. 2019.
Moran, Nick, et al. "Noisier2Noise: Learning to Denoise from Unpaired Noisy Data." Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition. 2020.
Mohan, Sreyas, et al. "Robust And Interpretable Blind Image Denoising Via Bias-Free Convolutional Neural Networks." International Conference on Learning Representations. 2019.
Figures