2185
Free-Breathing MR-based Attenuation Correction for Whole-Body PET/MR Exams1Siemens Healthcare GmbH, Erlangen, Germany, 2Department of Nuclear Medicine, University Hospital Ulm, Ulm, Germany, 3Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany
Synopsis
In whole-body PET/MR exams, MR-based attenuation correction is usually performed with a Dixon protocol of an MR VIBE sequence acquired in breath-hold followed by a segmentation into different tissue classes. As an extension we present a free-breathing approach for attenuation correction that can be used for patients that have problems or are even unable to perform the required breath-holds. The presented approach relies on a self-gated, compressed sensing accelerated gradient-echo sequence with Cartesian k-space sampling. We demonstrate the generation of free-breathing attenuation maps in 2 human volunteers and 10 patients.
Introduction
Whole-body PET/MR exams require the acquisition of an attenuation map for each single bed position to obtain quantitative PET images. Currently available MR-based attenuation correction (MRAC) methods usually rely on breath-hold acquisitions for bed positions that might be affected by respiratory motion. This approach leads to good results with cooperative patients. In cases where the patient cannot perform the breath-hold, resulting attenuation maps can suffer from artifacts. In patients unable to follow breath-hold commands, such as pediatric patients, this often leads to the need of anesthesia with the associated, known risks. In this work, we present a free-breathing approach for attenuation correction (FB-MRAC) based on a self-gated, compressed sensing accelerated gradient-echo sequence with Cartesian k-space sampling. FB-MRAC can be used in the critical bed positions, e.g. thorax and abdomen, which are heavily impacted by respiratory motion. The remaining bed positions can be acquired with the default MRAC and be composed resulting in whole-body free-breathing attenuation maps. In this study, we demonstrate the combination of free-breathing and breath-hold acquisitions of attenuation maps for whole-body PET/MR exams using human volunteers and patient data.Methods
All data were acquired on a 3T Biograph mMR (Siemens Healthcare, Erlangen, Germany) using the spine coil and body matrix coils. The breath-hold acquisitions were performed using a 2-point 3D VIBE Dixon prototype sequence with an acquisition time of 13s per volume. For the free‐breathing acquisitions, the same prototype sequence was used with an incoherent, variable‐density Cartesian k‐space sampling, integrated acquisition of a navigation signal, and a compressed‐sensing (CS) reconstruction with a total acquisition time of 113 seconds [1]. The navigation signal was used to sort the raw data into 4 motion states followed by the CS reconstruction. Only the end-expiration motion state was used to generate the attenuation map. The data were acquired for 2 healthy volunteers using three bed positions each and 10 patients with single bed positions only. All volunteers and patients provided written informed consent before the measurements. For the volunteer data, the first two bed positions were acquired with breath-hold and free-breathing respectively. The third bed position was acquired using the breath-hold setup but without a breath-hold command assuming that there is no severe respiratory motion. After the acquisition of the final bed position, the attenuation maps were generated using the bone segmentation [2] and the missing arms were added [3]. Detailed acquisition parameters were as follows: TR 3.96 msec; TE 1.23 / 2.46 msec; flip angle 10°; field‐of‐view 500 × 406.3 x 264 mm3; voxel size 1.3 × 1.3 × 3 mm3. The protocol with transversal acquisition and high in-plane resolution yielded Dixon images for clinical reading.Results
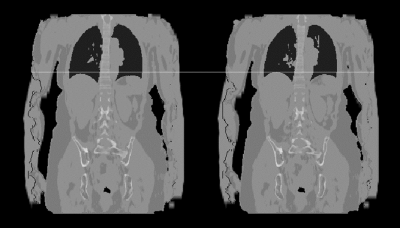
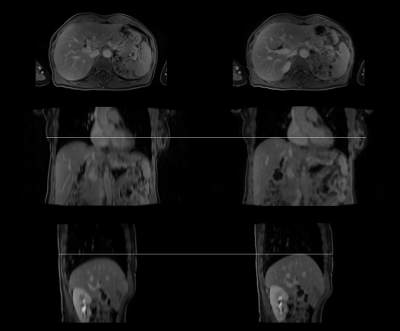
Figure 1 shows the comparison of whole-body attenuation maps of a healthy volunteer acquired in breath-hold and free-breathing respectively. The bone and lung segmentation worked successfully for both scan setups. In case of the free-breathing acquisition, the liver dome is closer to desired end-expiration state compared to the breath-hold acquisition. For both setups no artifacts are visible in the last bed position where motion was ignored. Figure 2 shows an example comparison of the Dixon water images acquired with breath-hold (left column) and during free breathing (right column) in three anatomical orientations. The transversal images show slightly different cross-sections of the liver due to the different respiratory positions. The images appear to have a similar sharpness. In case of the breath-hold acquisition the image impression of the liver is blurred in both coronal and sagittal reformats, especially towards the liver dome. In contrast to that the reformats appear sharper when using the free-breathing protocol.Discussion
For both patient and volunteer data the presented approach can provide free-breathing high-resolution Dixon images which can be used to generate PET attenuation maps. The advantage of this method can be seen in the patient example, where the patient cannot hold the breath for the entire duration of the required breath-hold leading to blurred images. As a drawback the new method requires longer reconstruction times due to the demanding CS reconstruction. It may require further evaluation whether there are cases where this method could fail or hamper the clinical evaluation. A future direction of improvement would be to investigate if acquisition time can be further reduced. The new approach could also be combined with PET motion correction techniques as e.g. discussed in [4].Conclusion
We demonstrated a method for free-breathing acquisition of high-resolution Dixon data suitable for the generation of attenuation maps for simultaneous PET/MR in approximately two minutes. This approach can be helpful in situations where breath-hold acquisitions are not possible and definitely lead to higher patient comfort if breath-holds can be avoided.Acknowledgements
No acknowledgement found.References
[1] Weiss et al., J. Magn. Reson. Imaging 2018;47:459–467
[2] Paulus et al., J Nucl Med 2015; 56:1061–1066
[3] Blumhagen et al., Medical Physics 41(2):022303
[4] Kolbitsch et al., Phys Med Biol. 2018 Jun 27;63(13)
Figures