2182
MR-based motion correction and anatomical guidance for improved PET image reconstruction in cardiac PET-MR imaging1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Nuklearmedizinische Klinik und Poliklinik, Technische Universitat Munchen, Munich, Germany, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4Department of Internal Medicine I, University hospital rechts der Isar, Technical University of Munich, Munich, Germany
Synopsis
Simultaneous PET-MR has shown promise for addressing several of the technical challenges that may degrade image quality in PET imaging, such as high noise levels, attenuation artefacts, and motion artefacts. While state-of-the-art PET image reconstruction techniques have addressed these issues separately, their combined effect has not been demonstrated. Here we introduce a single framework that integrates MR-based motion correction and anatomical guidance for improved simultaneous diagnostic cardiac PET-MR imaging. We evaluated the proposed framework on cardiac [18F]FDG PET-MR datasets and results show that, compared to conventional reconstruction algorithms, our framework results in sharper images, with increased contrast and reduced noise.
Introduction
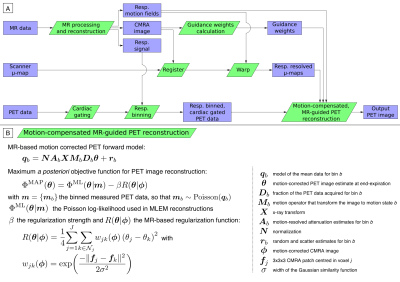
Simultaneous PET-MR has shown promise for addressing several factors that may result in degraded image quality in cardiac PET imaging. Previous studies have shown that MR-based motion correction reduces blurring and attenuation-induced artefacts, improving quantification and correspondence between both imaging modalities1-3. Additionally, MR-based anatomical guidance for regularized PET image reconstruction has been shown to improve PET image quality, with significant noise reduction while preserving edges4. This technique has been demonstrated mainly in brain PET-MR with promising results, but to the best of our knowledge, it has not been applied to cardiac PET-MR imaging, which is sensitive to motion.In this work we unite these two elements of state-of-the-art PET image reconstruction by developing a single framework that integrates MR-based motion correction and anatomical guidance in order to improve myocardial PET-MR imaging. We use a recently introduced sequence designed for simultaneous diagnostic cardiac PET-MR5, which provides both respiratory motion information and high-resolution motion-corrected MR images, that allows for cardiac PET images to be improved as follows: (1) attenuation maps (μ-maps) are aligned to the end-expiration respiratory position using MR images as reference to reduce attenuation-induced artefacts; (2) MR-derived motion information is incorporated into a motion-corrected image reconstruction (MCIR) of the PET data to reduce motion-induced blurring; and (3) high-resolution MR images enable anatomically guided PET image reconstruction, suppressing noise while preserving quantification performance. The proposed framework was evaluated on cardiac [18F]FDG PET-MR datasets and the effect of the different improvement steps in final image quality was investigated.
Methods
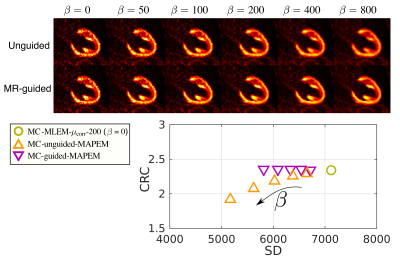
PET-MR data acquisition and MR image reconstruction: PET-MR data were acquired in a Siemens Biograph mMR scanner in 10 patients (age 59.3±12.3 y.o.; 8 male; 5 oncology patients without cardiac disease, 5 patients with known coronary artery disease) after injection of 343.7±25.3MBq of [18F]FDG as detailed in [5]. To summarize, acquired PET-MR data are binned into 4 respiratory bins for each patient based on 2D MR image navigators. The motion-corrected MR reconstruction results in a high-resolution end-expiration coronary MR angiography (CMRA) image with good contrast between blood pool and myocardial tissue, while additionally providing high-resolution respiratory motion fields. Relevant imaging parameters for the CMRA acquisition include coronal orientation, resolution=1×1×2mm3, field of view=304×304×88-112mm3 covering the whole heart, with data acquired during the mid-diastolic quiescent period of the cardiac cycle.MR-based PET image reconstruction: The proposed MR-based PET image reconstruction pipeline is as follows (Fig 1A): First, the standard μ-map is registered to the CMRA image to improve alignment between the attenuation factors and the PET image. Second, the μ-map is warped to each respiratory position using the MR-derived motion fields, in order to obtain respiratory-resolved μ-maps. Third, the CMRA image is used to calculate guidance weights for the PET image reconstruction6. Fourth, PET data is cardiac gated, with counts acquired outside of diastole discarded, and respiratory binned according to the MR-derived respiratory signal. Finally, this cardiac-gated respiratory-binned PET data, MR-derived guidance weights, MR-derived motion fields, and motion-resolved attenuation matrices are included in a respiratory motion-corrected cardiac-gated guided maximum a posteriori EM algorithm (Fig 1B). This algorithm (MC-guided-MAPEM) was run for 200 iterations using five different regularization strengths (β=50,100,200,400,800) and an automatic β selection method7.
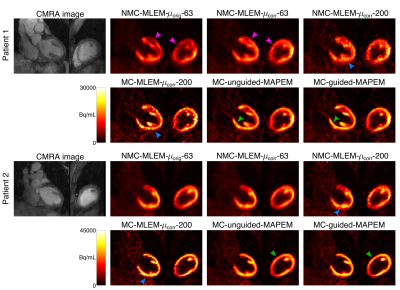
Comparative methods: To assess the impact of each component of the proposed framework, the following additional reconstructions were performed: (i) non-motion-compensated MLEM reconstruction using the scanner-provided μ-map, run for a clinically representative 63 iterations (NMC-MLEM-$$$\mu_\text{orig}$$$-63), to serve as starting point; (ii) non-motion-compensated MLEM using aligned μ-map, run for both 63 (NMC-MLEM-$$$\mu_\text{corr}$$$-63) and 200 iterations (NMC-MLEM-$$$\mu_\text{corr}$$$-200); to evaluate the effect of μ-map alignment and reconstruction convergence, respectively; (iii) a cardiac-gated respiratory motion-corrected MLEM (MC-MLEM-$$$\mu_\text{corr}$$$-200) using aligned μ-maps, run for 200 iterations; to evaluate the effect of motion compensation; and (iv) an unguided cardiac-gated respiratory motion-corrected MAPEM approach, where the guidance weights are replaced by spatially invariant weights that encourage a homogeneous smoothing (MC-unguided-MAPEM), run for 200 iterations with the same regularization parameter values as used for MC-guided-MAPEM; to evaluate the impact of MR-based anatomical guidance.
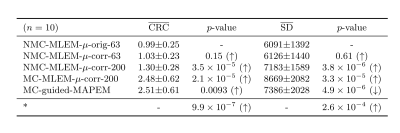
All images were quantitatively evaluated by measuring contrast recovery coefficient (CRC) between myocardium and blood pool, and the standard deviation (SD) in the myocardium was measured to provide a metric of image noise.
Results
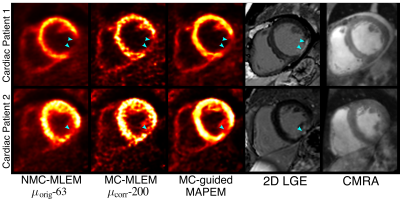
Fig 2 shows example slices for all comparative MR-based PET reconstruction methods for two oncology patients. MC-MLEM-$$$\mu_\text{corr}$$$-200 images show excellent contrast and delineation of the myocardium, however with high noise levels. Both unguided and MR-guided regularization reduce noise, but MR-guidance results in a better contrast/noise tradeoff (Fig 3). For cardiac patients, the proposed MC-guided-MAPEM method produces images with a good depiction of hypo-intense myocardial defects (Fig 4). These improvements result in a 154% increase in CRC compared to the conventional reconstruction (NMC-MLEM-$$$\mu_\text{orig}$$$-63) standard, and a 15% decrease in SD compared to motion compensation alone (Table 1).Conclusion
The proposed framework for MR-based motion compensation and anatomical guidance of cardiac PET data was shown to significantly improve image quality (contrast and noise) compared to alternative reconstruction methods. Furthermore, each step of the reconstruction pipeline was shown to have a positive effect in image quality. These improvements have the potential to improve clinical interpretability and diagnosis based on cardiac PET-MR.Acknowledgements
This work was supported by the following grants: (1) EPSRC EP/P032311/1, EP/P007619/1, EP/P001009/1; (2) BHF programme grant RG/20/1/34802 and (3) Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z).References
[1] R. Manber et al., “Joint PET-MR respiratory motion models for clinical PET motion correction,” Phys Med Biol, 2016, 61(17):6515-30
[2] C. Kolbitsch et al., “Respiratory-resolved MR-based attenuation correction for motion-compensated cardiac PET-MR,” Phys Med Biol, 2018, 63(13):135008
[3] P. M. Robson et al., “Correction of respiratory and cardiac motion in cardiac PET/MR using MR-based motion modeling,” Phys Med Biol, 2018, 63(22):225011
[4] G. Schramm et al., “Evaluation of Parallel Level Sets and Bowsher’s Method as Segmentation-Free Anatomical Priors for Time-of-Flight PET Reconstruction,” IEEE Trans. Med. Imaging, 2018, 37(2):590–603
[5] C. Munoz et al., “Motion-corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency,” Magn. Reson. Med., 2018, 79(1): 339-50
[6] S. Ellis et al., “Multi-Tracer Guided PET Image Reconstruction,” IEEE Trans. Radiat. Plasma Med. Sci., 2018, 2(5): 499–509
[7] A. J. Reader and S. Ellis, “Bootstrap-Optimised Regularised Image Reconstruction for Emission Tomography,” IEEE Trans. Med. Imaging, 2020, 39(6):2163-75
Figures